Chapter 16. The MAP Kinase Pathway in Yeast Mating
Introduction


Analyze the Data 16-2: The MAP Kinase Pathway in Yeast Mating
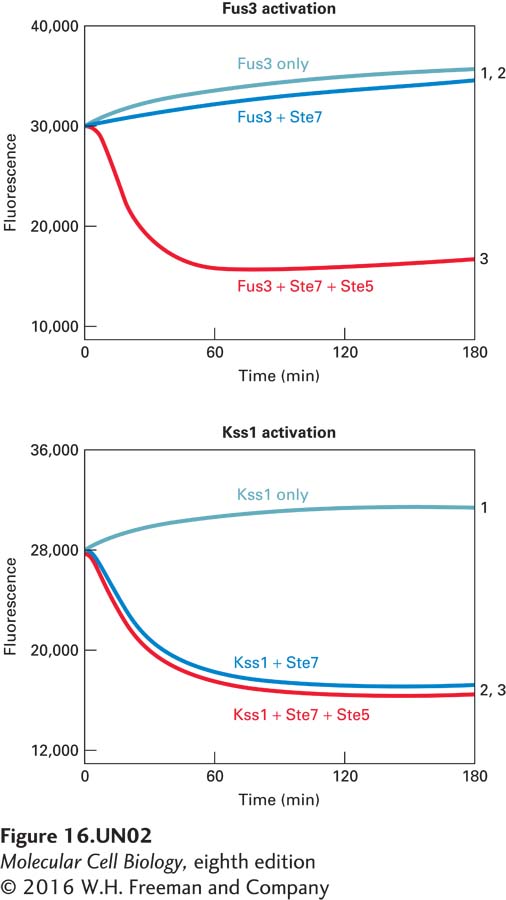
Scaffold proteins can segregate different MAP kinase signaling pathways that share common components. In the yeast mating pathway, the MEK Ste7 phosphorylates and activates the Fus3 MAP kinase, whereas Ste7 phosphorylates and activates the Kss1 MAP kinase in the starvation pathway. The mating pathway is activated by mating factor receptor activation of a G protein; Gβγ recruits the Ste5 scaffold protein and the Ste11, Ste7, and Fus3 components of the kinase cascade. Mutation of the Ste5 binding sites for Ste11 and Ste7 disrupts the mating response, clearly demonstrating the importance of Ste5 for tethering the kinases together. Mutation of the Fus3 binding site on Ste5 gives a more complicated response, suggesting that Ste5-Fus3 interaction may involve more than just tethering. This possibility was investigated with yeast proteins expressed as recombinant proteins in bacteria or insect cells and then purified. See Good et al., 2009, Cell 136:1085–1097.
a. A fluorescence quenching assay was used to measure the activity of the Fus3 and Kss1 MAP kinases using a substrate peptide that can be phosphorylated by both kinases. Phosphorylated peptide binds to gallium coupled to fluorescent beads and quenches the fluorescence. The rate of fluorescence quenching (loss of fluorescence) corresponds to the Fus3 or Kss1 kinase activity. Results of Ste7 phosphorylation, and thereby activation of Kss1 and Fus3, in the presence and absence of Ste5 are shown below:
1. Fus3 or Kss1 alone (control)
2. Fus3 or Kss1 + Ste7
3. Fus3 or Kss1 + Ste7 + Ste5
Is Ste5 required for Ste7 activity? Does Ste7 activate Fus3 and Kss1 equivalently? What does the effect of the presence or absence of Ste5 in the assay tell you about Ste7 phosphorylation and activation of Fus3 and Kss1?
b. The protein sequences of Fus3 and Kss1 are 55 percent identical, but each has a unique so-called MAP kinase activation loop near the activation domain that is phosphorylated by Ste7. Mutation of an isoleucine residue in the Fus3 insertion loop or replacement of the Fus3 insertion loop with the equivalent region of Kss1 both yield curves similar to Kss1 curve 2. What does this suggest about Fus3 and Kss1 as substrates for Ste7 and the role of Ste5 in stimulating Ste7 phosphorylation of Fus3?
Activity results are being submitted...