
Chapter 17. Regulation of Myosin V
Introduction

Analyze the Data 17-1: Regulation of Myosin V
Myosin V is an abundant nonmuscle myosin that is responsible for the transport of cargo such as organelles in many cell types. Structurally, it consists of two identical polypeptide chains that dimerize to form a homodimer. The motor domains reside at the N-terminus of each chain and contain both ATP- and actin-binding sites. The motor domain is followed by a neck region containing six “IQ” motifs, each of which binds calmodulin, a Ca2+-binding protein. The neck domain is followed by a region capable of forming coiled coils, via which the two chains dimerize. The final 400 amino acid residues form a globular tail domain (GTD), to which cargo binds. Myosin V would consume large amounts of ATP if its motor domain were always active, and a number of studies have been conducted to understand how this motor is regulated.
a. The rate of ATP hydrolysis (i.e., ATP molecules hydrolyzed per second per myosin V) was measured in the presence of increasing amounts of free Ca2+. The concentration of cytosolic free Ca2+ is normally less than 10−6 M, but can be elevated in localized areas of the cell and is often elevated in response to a signaling event. What do these data suggest about myosin V regulation?
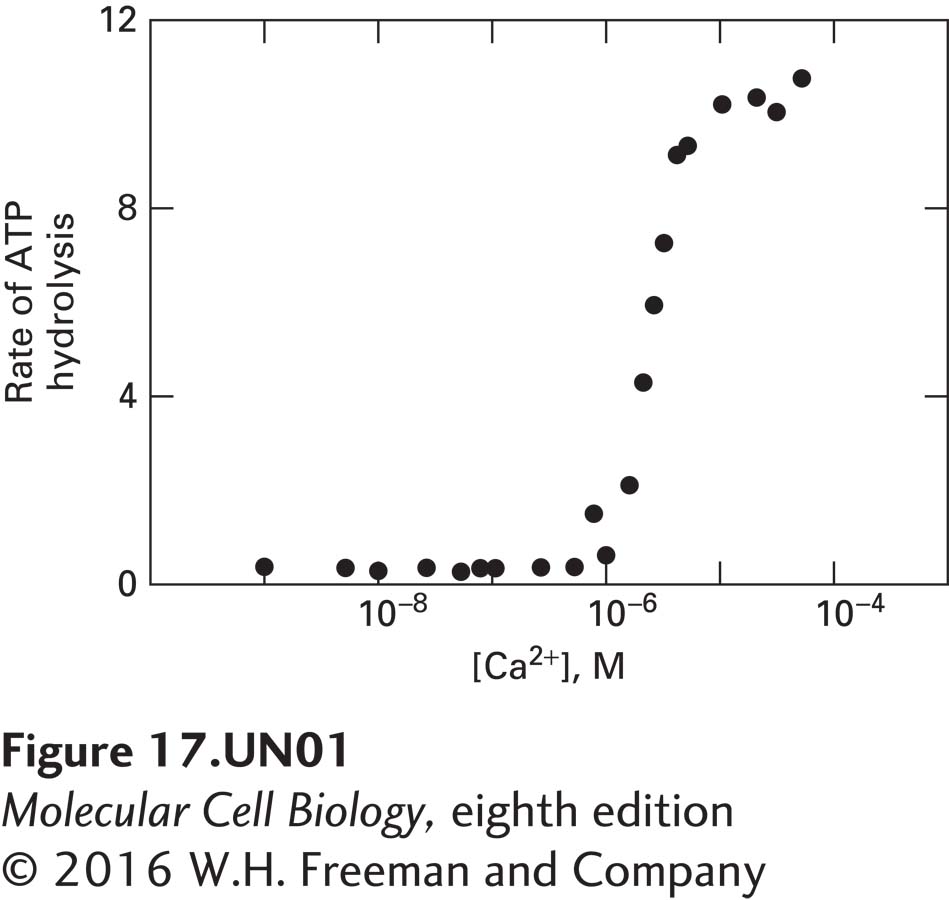
These data show that myosin V has low ATPase activity when the free Ca2+ is below 1 micromolar. Thus, one would expect that myosin V would be inactive except under conditions in which the cytosolic free Ca2+ were elevated above this value, perhaps in localized domains of the cell or upon excitation of excitable cells.
b. In additional studies, the ATPase activity of myosin V was measured in the presence of increasing amounts of F-actin in the presence or absence of 10-6 M free Ca2+. What additional information about myosin V regulation do these data provide?
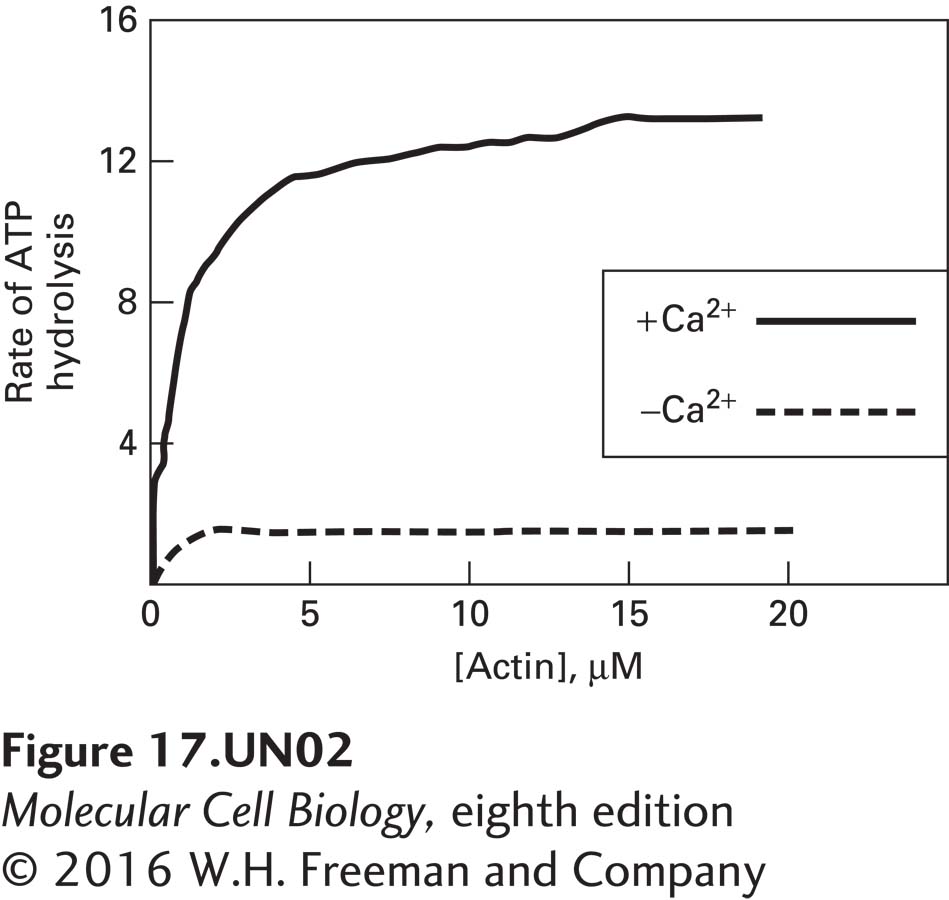
These data reveal that myosin V has low ATPase activity in the absence of actin, which suggests that, in vivo, myosin V does not hydrolyze ATP at significant rates unless it can bind to and move along actin filaments. The actin-activated ATPase activity is also significantly repressed at low Ca2+. Thus, two criteria may need to be met in vivo for myosin V to hydrolyze ATP. The cytosolic free Ca2+ concentration must become elevated above 1 micromolar, and there must be actin filaments with which the myosin V can functionally interact.
c. Next the behavior of truncated myosin V, which lacks only its C-terminal globular tail, was examined and compared with the behavior of intact myosin V. From this experiment, what can you deduce about the mechanism by which myosin V is regulated?
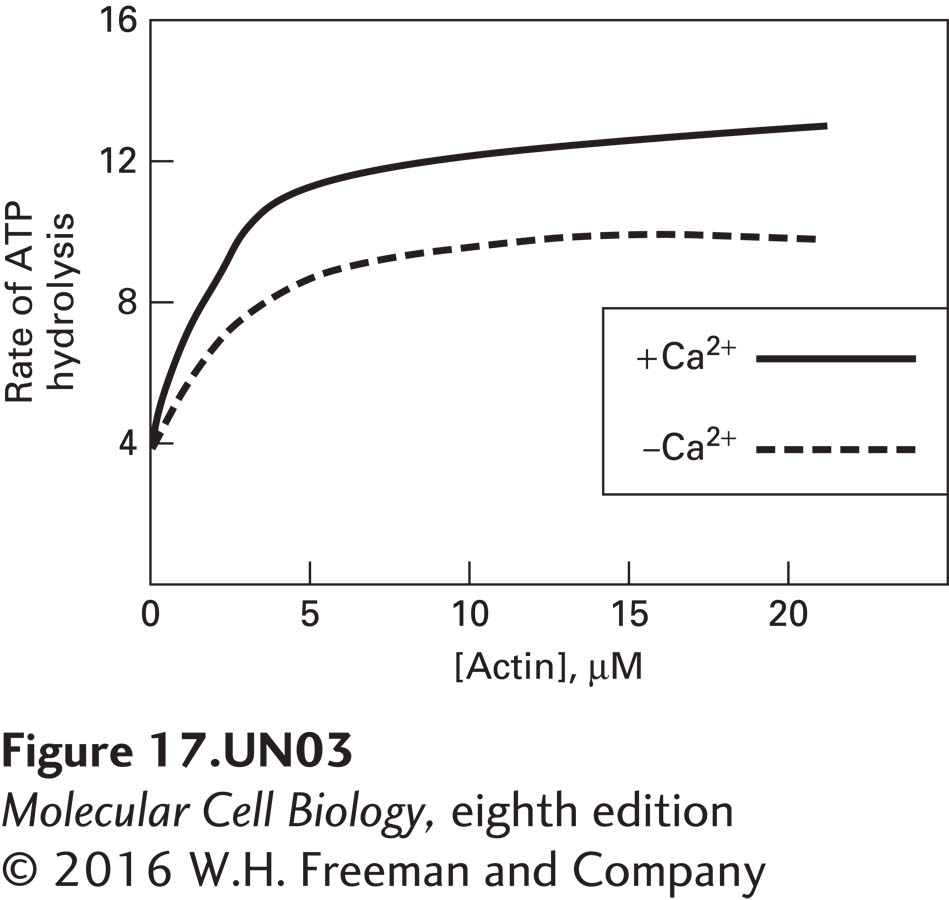
Unlike intact myosin V, in which the ATPase activity is inhibited in the absence of Ca2+, truncated myosin V, lacking its globular tail domain, exhibits actin activated ATPase activity even in the absence of Ca2+. These data suggest that the tail domain of myosin V may be required to keep the myosin in an inactive state in the absence of Ca2+.
Activity results are being submitted...