
Chapter 20. E-Cadherin Isoforms and Function
Introduction

Analyze the Data 20-1: E-Cadherin Isoforms and Function
Researchers have isolated two mutant E-cadherin isoforms that are hypothesized to function differently from wild-type E-cadherin. An E-cadherin-negative mammary carcinoma cell line was transfected with the mutant E-cadherin genes A (part a in the figure; triangles), or B (part b; triangles) or the wild-type E-cadherin gene (filled circles) and compared with nontransfected cells (open circles) in an aggregation assay. In this assay, cells were first dissociated by trypsin treatment and then allowed to aggregate in solution over a period of minutes. To demonstrate that the observed adhesion was mediated by cadherin, the cells were pretreated with a nonspecific antibody (left panels) or a function-blocking anti-E-cadherin monoclonal antibody (right panels).
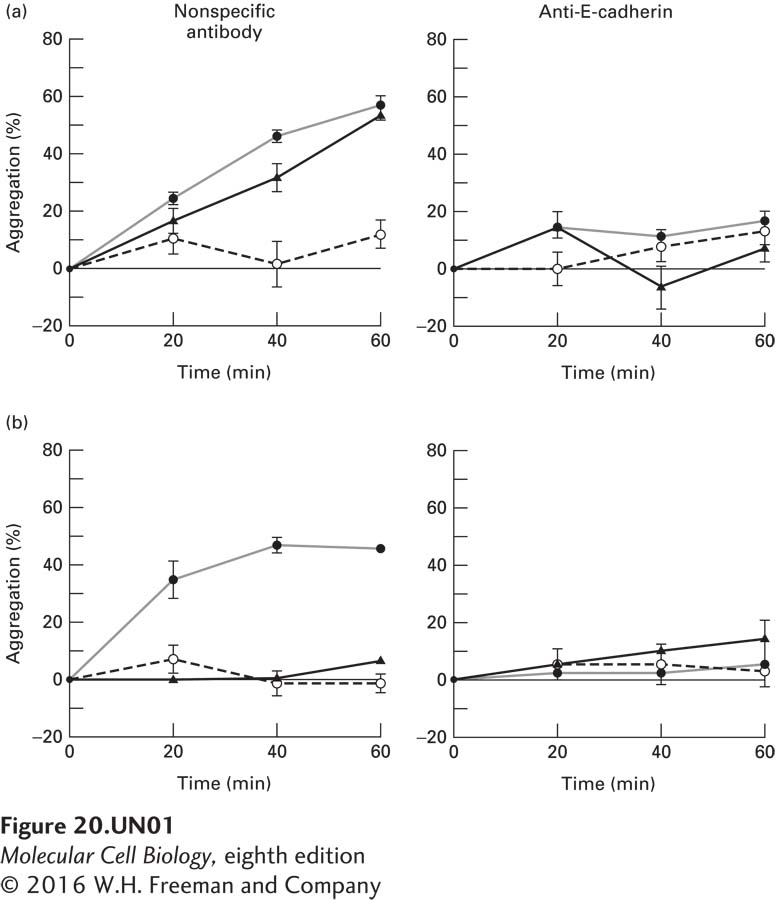
a. Why do cells transfected with the wild-type E-cadherin gene have greater aggregation than control, nontransfected cells?
Cells transfected with wild-type E-cadherin aggregate more than untransfected cells because the increase in E-cadherin allows for more cell-cell interactions via the ECM.
b. From these data, what can be said about the function of mutants A and B?
Mutant A behaves almost identically to the wild-type E-cadherin in the aggregation assay; therefore, this mutation does not change the function of E-cadherin as far as hemophilic interactions are concerned. Expression of mutant B, however, does not result in increased aggregation, so this particular mutation does alter the adhesive qualities of E-cadherin.
c. Why does the addition of the anti-E-cadherin monoclonal antibody, but not the nonspecific antibody, block aggregation?
The monoclonal antibody specific for E-cadherin blocks the resulting aggregation because it binds to E-cadherin and blocks homophilic inter-actions. In contrast, the nonspecific antibody does not specifically interact with E-cadherin, and does not block homophilic interactions.
d. What would happen to the aggregation ability of the cells transfected with the wild-type E-cadherin gene if the assay were performed in media low in Ca2+?
Since cadherins require calcium for function, lowering the calcium in the media during the assay would lower the aggregation ability.
Activity results are being submitted...