 Overall Progress: 0%
Overall Progress: 0%

Chapter 1. Alzheimer's Disease
1.1 Introduction
Alzheimer's Disease
Protein Folding Case Study
Popup content goes in this box
New box content

Dr. Walter Thomas, founder and chief scientific officer of iStem, pulls into his parking stop early on a brisk, fall Saturday morning. After obtaining a PhD in Cellular and Molecular Biology, Dr. Thomas took a postdoctoral position at the National Institutes of Health to focus on drug discovery using stem cell technologies. Most of his training in the 1990s focused on the use of mouse embryonic stem cells (ESCs), discerning what cocktail of growth factors and culture conditions could be used to coax these undifferentiated, pluripotent cells into a given differentiated cell type. Heart cells, liver cells, neurons, and muscle cells all had differing preferences in what needed to be included in the culture medium.
Then human ESCs became available in the late 1990s, and many of the same differentiation protocol recipes still held true of both mouse and human ESCs, with some slight modifications here and there. Few people had banked ESCs and so the potential of personalized medicine through human ESCs was still mostly unrealized, but the discovery of induced pluripotent stem (iPS) cells in 2007 was a game changer. Now it was possible to make iPS cells directly from the somatic cells of the patient.
Dr. Thomas soon switched the focus of his company from using human ESCs to making iPS cells, which could be generated from patients with a particular disease. The goal of his company was to generate disease-specific iPS cells, differentiate those cells into the disease-appropriate cell type, and then use a drug screening protocol to test for effective therapeutics. Two drugs, now in clinical trials to treat different heart conditions, looked extremely promising based on the data. After years of cardiovascular research, Dr. Thomas was seemingly seeing the light at the end of the tunnel. This morning, however, he was looking at expanding his company in a different area. He opened the folder labeled "Neuroscience Search" and started reviewing applicants for his company's job openings.
The previous summer, Dr. Thomas lost his mother to Alzheimer's disease, and during her decline in health he realized the impact a disease has on both the patient and the family. Since his mom’s diagnosis, his main focus was on making sure that she was taken care of; she often stayed for extended periods with him and his siblings. After the funeral, his focus shifted to concerns about what the future held for him, his siblings, and in particular, his children. Dr. Thomas wondered whether there was a genetic component that made them predisposed to Alzheimer’s. Any time he misplaced his keys or had a lapse in memory, he wondered if these were early warning signs.
He wanted to assemble a team of neuroscientists that could help with the drug discovery, gene therapy, and stem cell–based approaches to treat or slow the progression of Alzheimer's disease. Although his background training was not in the neurosciences, he felt a sense of urgency to personally find out more about the genetics, molecular causes, and treatment approaches for Alzheimer’s disease.
1.2 Symptoms and Disease Progression
Symptoms and Disease Progression
According to the Alzheimer's Association, there are 10 early signs and symptoms of Alzheimer's disease, and if any are noticed then one should schedule an appointment with a doctor. These signs can be found at: http://www.alz.org/alzheimers_disease_10_signs_of_alzheimers.asp
1.
A few years ago Dr. Thomas attributed some of his mother's forgetfulness to getting older, but once the signs became more apparent he was not surprised of the diagnosis.
According to the Alzheimer’s Association, there are typical age-related changes in memory which are distinct from signs from Alzheimer’s disease. Which of the following would be a typical age-related change?
| A. |
| B. |
| C. |
| D. |
| E. |
2.
Dr. Thomas has written down some of his symptoms over the past year. These include: (1) making a bad decision once in a while; (2) forgetting which day it is and remembering later; (3) losing things from time to time; and (4) missing a monthly payment once in a while. Does Dr. Thomas suffer from typical age-related changes or Alzheimer’s disease?
| A. |
| B. |
Correct.
Alzheimer’s disease progresses at different rates, with most people living 4 years to 8 years after diagnosis. There are three different stages, from mild (early stage) to moderate (middle stage) to severe (late stage). The common difficulties and signs associated with each stage can be found at http://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp.
Incorrect.
Alzheimer’s disease progresses at different rates, with most people living 4 years to 8 years after diagnosis. There are three different stages, from mild (early stage) to moderate (middle stage) to severe (late stage). The common difficulties and signs associated with each stage can be found at http://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp.
3.
What stage of Alzheimer’s disease would correspond to forgetting material that one just read and losing or misplacing a valuable object?
| A. |
| B. |
| C. |
4.
What stage of Alzheimer’s disease would correspond to losing awareness of recent experiences as well as of their surroundings, and experiencing changes in physical abilities, including the ability to walk, sit and, eventually, swallow?
| A. |
| B. |
| C. |
5.
What stage of Alzheimer's disease would correspond to feeling moody or withdrawn, especially in socially or mentally challenging situations, and forgetfulness of events or about one's own personal history?
| A. |
| B. |
| C. |
1.3 Genetics
Genetics
There are two major types of Alzheimer’s disease: (1) Early-onset, or familial, Alzheimer’s disease (FAD), which occurs generally between the ages of 30 years and 60 years and represents 5% of cases, and (2) late-onset Alzheimer’s disease, which usually occurs after the age of 65. To understand the risk factors involved, follow the links: https://www.nia.nih.gov/alzheimers/publication/alzheimers-disease-genetics-fact-sheet and http://www.alz.org/alzheimers_disease_causes_risk_factors.asp.
6.
Based on the function of presenilin 1, what cellular process would you expect to be altered from mutations in the presenilin 1 gene?
| A. |
| B. |
| C. |
| D. |
| E. |
7.
Dr. Thomas’s mother was diagnosed with Alzheimer’s disease at age 72. Which gene should Dr. Thomas test for Alzheimer’s disease-causing mutations?
| A. |
| B. |
| C. |
| D. |
| E. |
8.
Dr. Thomas gets tested and finds that he has an apolipoprotein E4 (ApoE4) allele variant that puts him at an increased risk for Alzheimer’s disease. What variant does he have?
Answer options:
| A. |
| B. |
| C. |
Those people who have a parent, brother, sister, or child with Alzheimer’s are more likely to develop the disease. The risk increases if more than one family member has the illness. When diseases tend to run in families, either genetics or environmental factors, or both, may play a role. In terms of genetic risks, families that have FAD have higher risks from variations in the genes encoding for amyloid precursor protein (APP), presenilin 1 (PS-1) and presenilin 2 (PS-2). For late-onset Alzheimer’s disease the risk gene with the strongest influence is apolipoprotein E4. There are three known allele variants, and the allele linked to Alzheimer’s disease is APOE-ε4, a possible factor in 20 to 25 percent of Alzheimer's cases. The APOE-ε2 allele confers protection against Alzheimer’s disease, while the APOE-ε3 allele is neutral. Individuals who are homozygous for the APOE-ε4 allele have an increased risk for Alzheimer’s disease compared with heterozygotes, who have an increased risk over individuals who don’t carry any copies of the APOE-ε4 allele.
A more in-depth analysis of Dr. Thomas’s family revealed that his mother had two APOE-ε4 alleles, while he and his siblings each only have one APOE-ε4 allele. Dr. Thomas was informed by his physician that people with two APOE-ε4 alleles don’t necessarily get Alzheimer’s disease, and those who get Alzheimer’s disease don’t necessarily have an APOE-ε4 allele. There are also additional genetic and environmental factors. While one of nine people 65 years or older has Alzheimer's, nearly one in three people 85 years or older has the disease. One of the greatest mysteries of Alzheimer's disease is why risk rises so dramatically as we grow older.
9.
Propose a hypothesis to explain why aging might cause an increased incidence of Alzheimer’s disease.
1.4 Pathology
Pathology
In reading the scientific literature, Dr. Thomas found that Alzheimer’s disease arises from the loss of neurons in the neocortex from proteinopathies that arise both inside and outside the cell. Sticky protein aggregates, known as amyloid plaques, develop outside the neurons, while inside the cell there is the formation of neurofibrillary tangles from alterations in microtubule stability. Brains from patients with Alzheimer’s disease classically have these hallmark pathological signs. Due to the amyloid plaques and neurofibrillary tangles throughout the brain, healthy neurons stop functioning, lose connections with other neurons, and die. The damage initially appears to take place in the hippocampus, the part of the brain essential in forming memories. As more neurons die, additional parts of the brain are affected, and they begin to shrink. By the final stage of the disease, damage is widespread, and brain tissue has shrunk significantly.
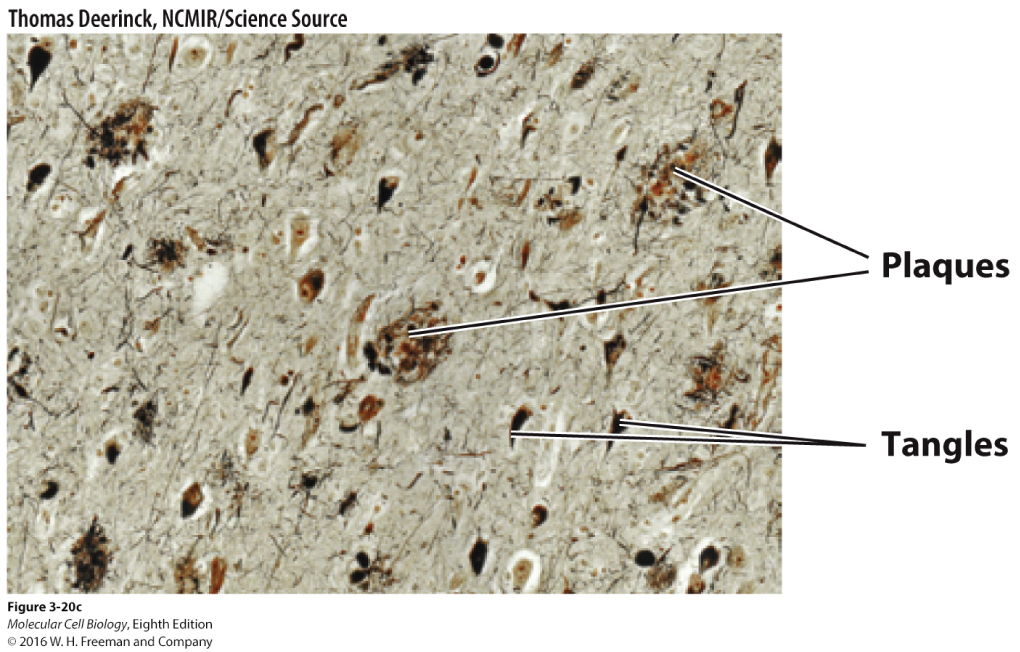
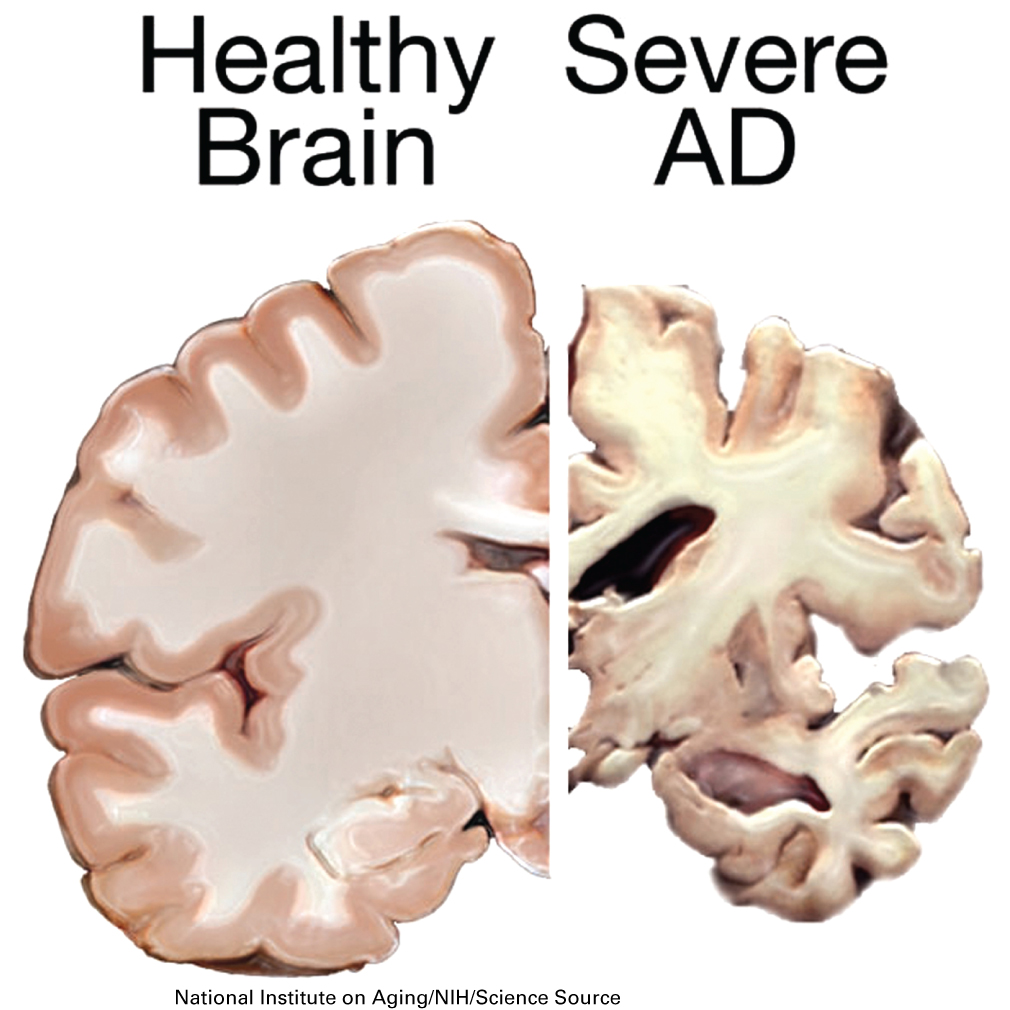
10.
Being a naturally curious person, Dr. Thomas wondered how neurofibrillary tangles and amyloid plaques develop and ultimately contribute to Alzheimer’s disease. He started by looking into what happens inside the cell that causes neurofibrillary tangles to develop.
Neurofibrillary tangles arise from microtubule instability due to problems associated with tau proteins. What role do tau proteins play with regard to microtubule stability?
| A. |
| B. |
| C. |
| D. |
| E. |
11.
Tau proteins are MAPs, or microtubule-associated proteins. In the development of neurofibrillary tangles found in Alzheimer’s disease, which of the following statements is true?
| A. |
| B. |
| C. |
| D. |
| E. |
Tau proteins are expressed in neuronal cells and act to stabilize microtubules and also act as spacers between the microtubules.
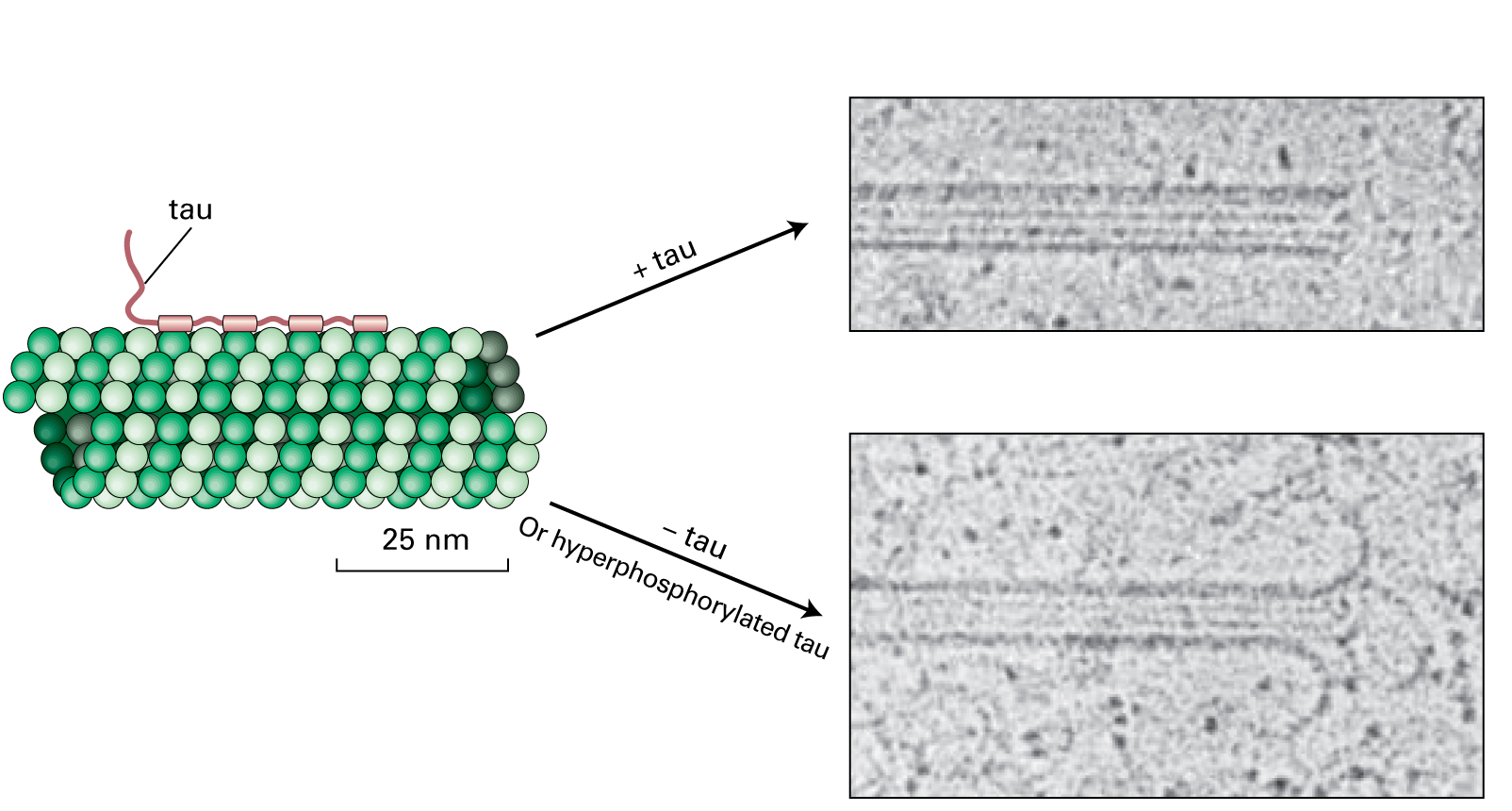
When tau proteins become hyperphosphorylated they self-aggregate to form tau filaments or neurofibrillary tangles in the cytosol of the neurons. Destabilization and loss of microtubules would result in a decrease in cell transport, needed for neurotransmitter localization at the synaptic terminus, and a loss of cell-cell communication.
Proteinopathies are diseases that arise from protein misfolding, and examples of diseases in this class include Creutzfeldt-Jakob disease, Alzheimer’s disease, Parkinson’s disease, and prion protein diseases. Hyperphosphorylated tau proteins associate as protein clumps, and this pathology of Alzheimer’s disease makes it a proteinopathy. Cells normally degrade misfolded proteins, but chronic expression of misfolded proteins can lead to the formation of protein aggregates or plaques. These protein aggregates or plaques can be harder for the cell to clear, which can inhibit normal cellular function and ultimately prove to be toxic.
12.
What mechanism does the cell use to remove or degrade individual misfolded proteins?
| A. |
| B. |
| C. |
| D. |
| E. |
Correct.
Individual proteins are tagged by poly-ubiquitination at lysine residues, and then routed to the proteasome for degradation. Autophagy can also be used to remove protein aggregates found in the cytosol. These two companion systems are responsible for the processing of long-lived proteins, misfolded proteins, and excess proteins. Unfortunately, autophagy levels decline as we age, making us more susceptible to proteinopathies.
Incorrect.
Individual proteins are tagged by poly-ubiquitination at lysine residues, and then routed to the proteasome for degradation. Autophagy can also be used to remove protein aggregates found in the cytosol. These two companion systems are responsible for the processing of long-lived proteins, misfolded proteins, and excess proteins. Unfortunately, autophagy levels decline as we age, making us more susceptible to proteinopathies.
In contrast to intracellular neurofibrillary tangles, plaques form extracellularly through the processing of the amyloid precursor protein (APP). Plaques are hallmark signs in Alzheimer’s disease and occur in most cases, but are only confirmed microscopically postmortem. Although not completely understood, it is thought that protein components of these plaques may engage death receptors on the surfaces of adjacent neurons.
Figure 16-37a shows the processing of APP.
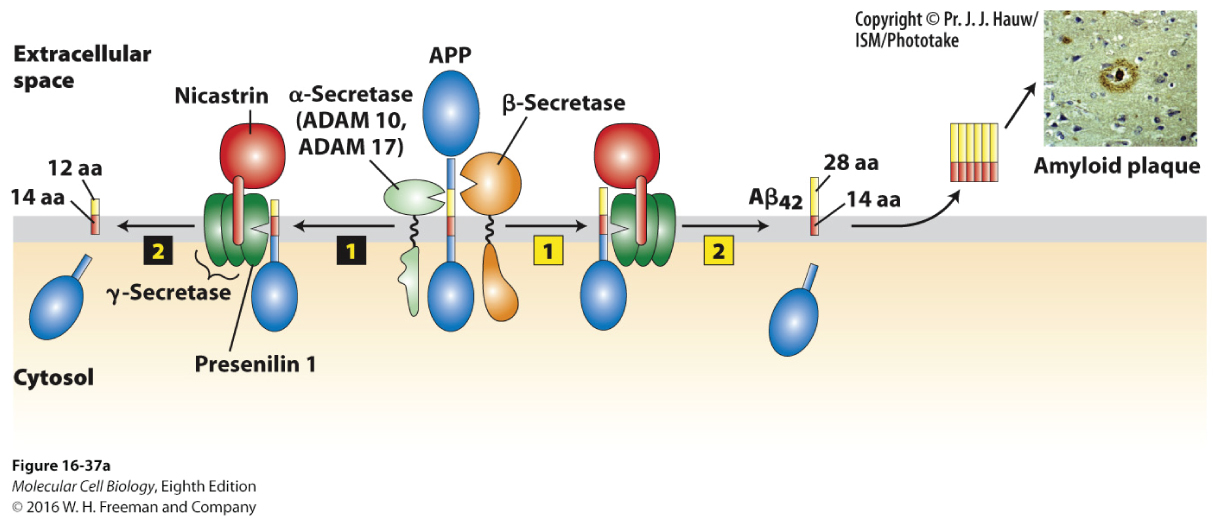
Processing of APP by α-secretase then by γ-secretase produces a 26–amino acid membrane–bound peptide. However, processing by β-secretase followed by γ-secretase generates a 40–amino acid peptide, Aβ40. Mutation within the gene encoding presenilin 1, a component of the γ-secretase complex, is a predisposing genetic risk factor for Alzheimer’s disease. Under these conditions, the cell generates a 42–amino acid peptide, Aβ42, which forms oligomers and then subsequent amyloid plaques.
13.
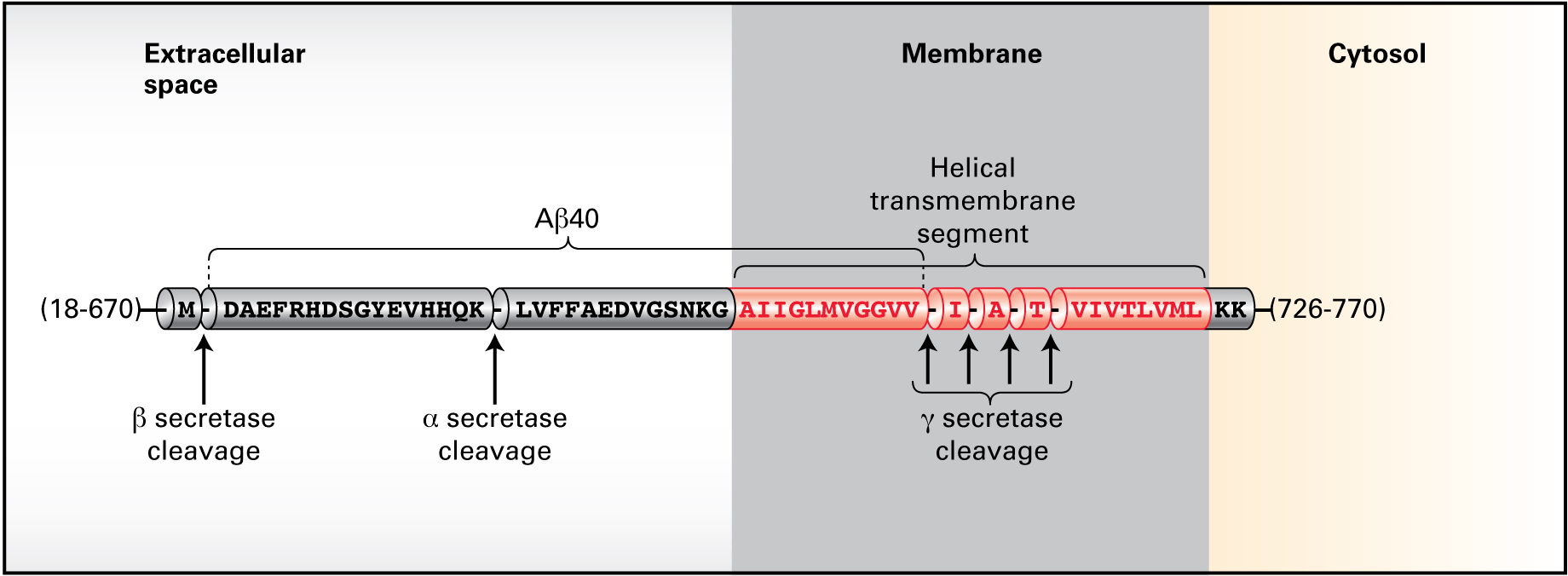
Given the end of the Aβ40 peptide is the transmembrane segment AIIGLMVGGVV shown above, how would the two extra hydrophobic amino acids in the Aβ42 peptide influence protein aggregation?
1.5 Treatment
Treatment
Finding no clear way to know for sure whether he or his relatives will develop Alzheimer’s disease, Dr. Thomas moved on to possible treatment options. Treatment strategies have focused on three possible avenues: (1) prevent Aβ42 peptide formation; (2) remove the Aβ42 peptide once formed, and (3) prevent Aβ42 peptide interaction.
14.
Transgenic mice that expressed a mutant version of the human APP gene were injected with a vaccine for the aggregated Aβ42 peptide. Injections occurred at 6 weeks of age (before plaque deposition occurs), and on a monthly basis for 11 months. High titers of anti-Aβ antibodies developed, and at 12 months of age, the mice showed dramatic reductions in plaque loads in comparison with saline-injected control mice. What type of approach is this considered?
| A. |
| B. |
Passive immunotherapy relies on the direct injection of antibodies, which would target a known protein. Active immunotherapy is based on the stimulation of one’s own immune system to raise antibodies against the foreign peptide.
Based on this and similar preclinical trials, active immunotherapy entered clinical trials. Clinical trials are classified by stages and each stage can take several years. Phase I trials: Researchers test a new drug or treatment in a small group of people for the first time to evaluate its safety, determine a safe dosage range, and identify side effects. Phase II trials: The drug or treatment is given to a larger group of people to see if it is effective and to further evaluate its safety. Phase III trials: The drug or treatment is given to large groups of people to confirm its effectiveness, monitor side effects, compare it with commonly used treatments, and collect information that will allow the drug or treatment to be used safely. Phase IV trials: Studies are done after the drug or treatment has been marketed to gather information on the drug's effect in various populations and any side effects associated with long-term use.
Phase I trials of the Aβ42 peptide vaccine in humans showed no ill effects, but a larger phase II trial showed that several patients developed inflammation in the brain when using the Aβ42 peptide vaccine approach. Use of a passive immunization approach by intravenous injection of Aβ42 antibodies into patients went through a Phase III trial, but the results were disappointing and showed no signs of slowing the progression of the disease. Antibody delivery across the blood-brain barrier utilizes the transferrin receptor pathway as a shuttle system to facilitate delivery into the brain.
Another avenue is to inhibit the processing of APP, and several BACE1 (β-site amyloid precursor protein cleaving enzyme 1) inhibitors target the β-secretase enzyme. Initial problems showed difficulty in getting inhibitors across the blood-brain-barrier, but this issue has been resolved and clinical trials are underway to determine safety. Currently there are more than 30 known substrates for BACE1, so there could be unintended side effects from the use of inhibitors, although side effects may vary with the dosage used.
15.
What signaling pathway is dependent upon the processing capability of BACE1?
| A. |
| B. |
| C. |
| D. |
| E. |
16.
Notch/Delta signaling is important from a developmental standpoint in a process termed lateral inhibition, where adjacent and developmentally equivalent cells can assume completely different developmental fates. How does this occur?
| A. |
| B. |
| C. |
| D. |
| E. |
17.
In the responding cell, the released intracellular Notch domain acts as a transcriptional activator to effectively increase production of Notch and downregulate Delta. The cell produces more Notch and less Delta than its neighbors, entering a Notch/Delta signaling loop that ultimately causes the cell to assume a different fate than the adjacent cells.
How can Dr. Thomas use his stem cell company to profile the progression of Alzheimer’s disease and be a resource to test pharmaceuticals?
| A. |
| B. |
iPS cells can be generated from skin biopsies from patients suffering from Alzheimer’s disease, differentiated into neurons, and then characterized in vitro. They also can be used to test the efficacy of different drugs in a high throughput screen. Labs have already generated iPS cells from patients having APP, presenilin 1, and presenilin 2 mutations. Dr. Thomas’s company could isolate additional iPS cell lines that carry different mutations in these genes, but also develop lines that carry different APOE4 alleles (APOE-ε2; APOE-ε3; APOE-ε4) for which he and his family could provide somatic lines for their derivation. Considerable future work will focus on improving the in vitro differentiation systems and enhancing the maturity and network connectivity of neurons in vivo.
1.6 Additional Information
Additional Information: Alzheimer’s disease
Treatment of Alzheimer’s Disease
Alzheimer’s disease is complex, and it is unlikely that any one drug or other intervention can successfully treat it. Current approaches focus on helping people maintain mental function, manage behavioral symptoms, and slow or delay the symptoms of disease. The major problem with the treatment of multifactorial diseases, which arise from the influences of multiple genes and the environment, is that they are not always amenable to therapeutic intervention. The complexity of neuronal function and integration, as well as the inherent difficulties in replacing diseased and lost neurons, only compounds the challenge of finding a cure. As more is uncovered about the basic biology underlying neuronal dysfunction, researchers hope to develop new therapies that target specific genetic, molecular, and cellular mechanisms so that the actual underlying cause of the disease can be stopped or prevented one day.
- Maintaining Mental Function: Several medications are approved by the U.S. Food and Drug Administration to treat symptoms of Alzheimer’s disease. Donepezil (Aricept), rivastigmine (Exelon), and galantamine (Razadyne) are used to treat mild to moderate Alzheimer’s (donepezil can be used for severe disease as well). Memantine (Namenda) is used to treat moderate to severe Alzheimer’s disease. These drugs work by regulating neurotransmitters, the chemicals that transmit messages between neurons. They may help maintain thinking, memory, and communication skills, and help with certain behavioral problems. However, these drugs don’t change the underlying disease process. They are effective for some but not all people and may help only for a limited time.
- Managing Behavior: Common behavioral symptoms of Alzheimer’s disease include sleeplessness, wandering, agitation, anxiety, and aggression. Scientists are learning why these symptoms occur and are studying new treatments—drug and nondrug—to manage them. Research has shown that treating behavioral symptoms can make people with Alzheimer’s disease more comfortable and makes things easier for caregivers.
- Looking for New Treatments: Alzheimer’s disease research has developed to a point where scientists can look beyond treating symptoms to think about addressing underlying disease processes. In ongoing clinical trials, scientists are developing and testing several possible interventions, including immunization therapy, drug therapies, cognitive training, physical activity, and treatments used for cardiovascular and diabetes. For example, the Systolic Blood Pressure Intervention Trial (SPRINT) is evaluating the health effects of lowering systolic blood pressure, while the add-on study, called SPRINT-MIND, is specifically examining the effects of lower systolic blood pressure on cognitive decline and development of Alzheimer’s disease.
https://www.nia.nih.gov/alzheimers/publication/alzheimers-disease-fact-sheet#changes

Your grade of 0% has been submitted.