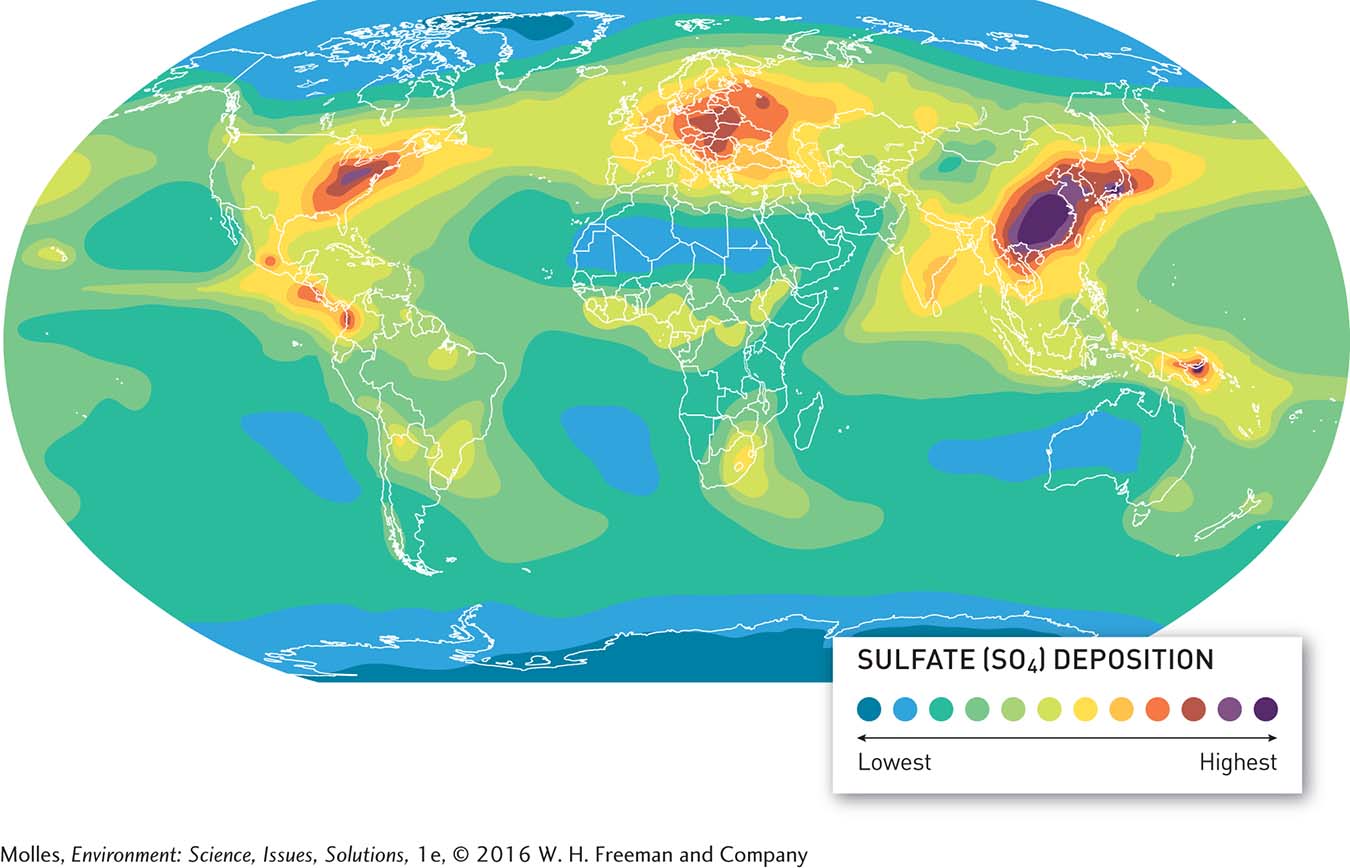
13.5 Acid rain is a major source of damage to aquatic and terrestrial ecosystems
From the beginning, almost everyone knew who was to blame for the acid rain in Sudbury, Ontario. In 1918 a group of farmers there sued and later won a lawsuit against the Canadian Copper Company for damage done to their crops by fumes from the company’s smelting operations. Nevertheless, broader recognition of the problem of acid rain would take another century as scientists started documenting highly acid rains far from population centers and because of its conspicuous damage to forests.
How Acid Rain Harms Ecosystems
Beginning in the mid-

base A substance that has the capacity to neutralize acids; bases release hydroxide ions (OH−) and react with acids to form a salt and water.
buffering capacity A measure of the ability of a solution to neutralize acid.

Who should compensate for damage to ecosystems by acid rains?
Regions such as the northeastern United States, much of Canada, and Scandinavia are particularly vulnerable because their soils have a low capacity for buffering the effects of acids as a result of their low base content. A base is a substance that has the capacity to neutralize acids. In general, buffering capacity is a measure of the ability to neutralize acid. Buffering capacity is higher for soils, or waters, with higher base concentrations because they can absorb more acid without changing pH. Soils in other regions, such as the U.S. Midwest and Southwest, generally have higher base content and thus a greater capacity to neutralize acids; they are therefore less threatened by acid rain.
Acid rain harms trees by depleting soil nutrients, which are dissolved and washed away in acidic soil water. Furthermore, acid rain releases aluminum from soils, which is toxic to plants in high enough concentrations. Consequently, with long exposure to acid rain, forest soils are slowly stripped of essential plant nutrients and can become increasingly toxic. Exposure to acidic clouds and fogs can also cause damage to the leaves and needles of trees. Plants stressed by such conditions become vulnerable to a number of other potential sources of mortality, including insects, disease, drought, and cold.
Impacts on Aquatic Ecosystems
Acid rain also impacts organisms that live in water. When acidic water seeps into soils and infiltrates the shallow groundwater, it releases aluminum. When this runoff water flows into streams and lakes, its pH is low and its aluminum content is high—

What criteria would you use to choose organisms as indicators of the impacts of acid rain on aquatic or terrestrial ecosystems?
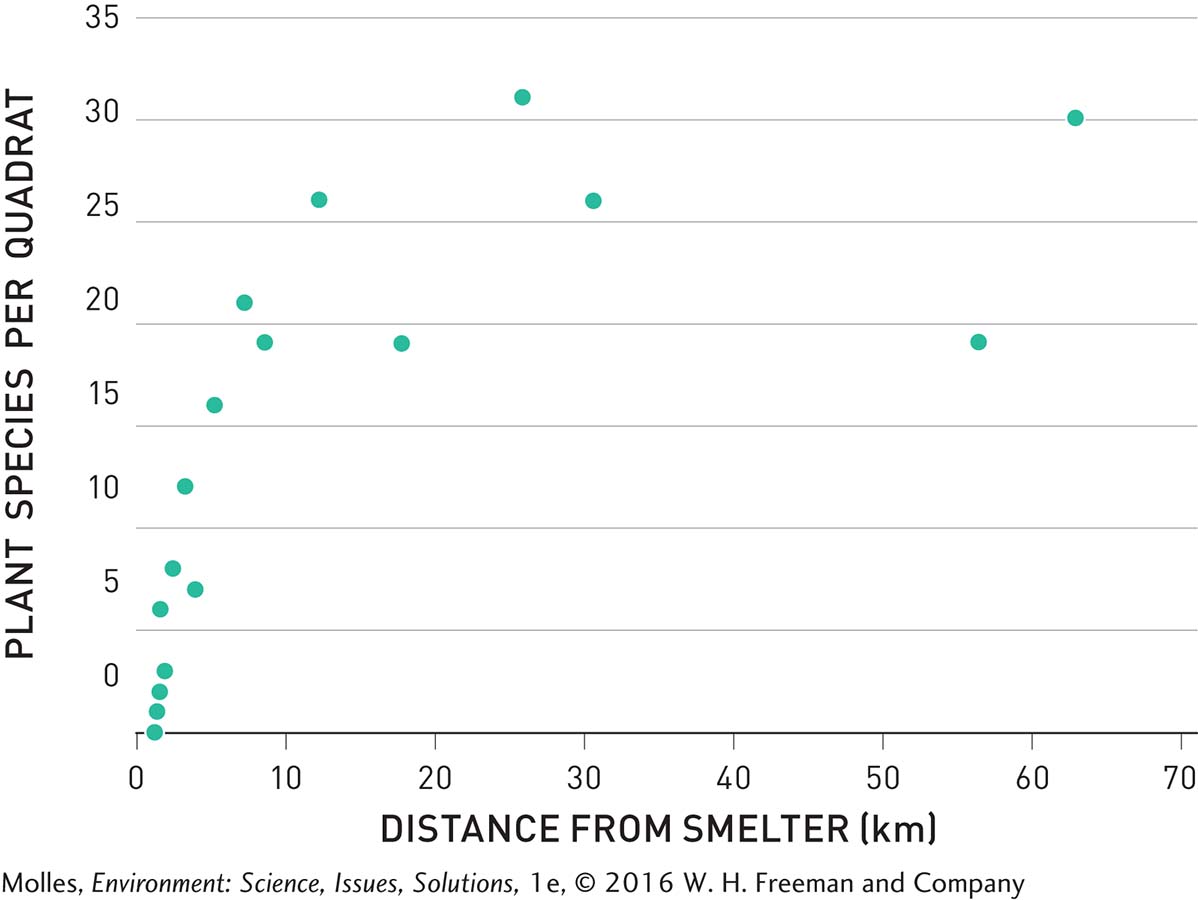
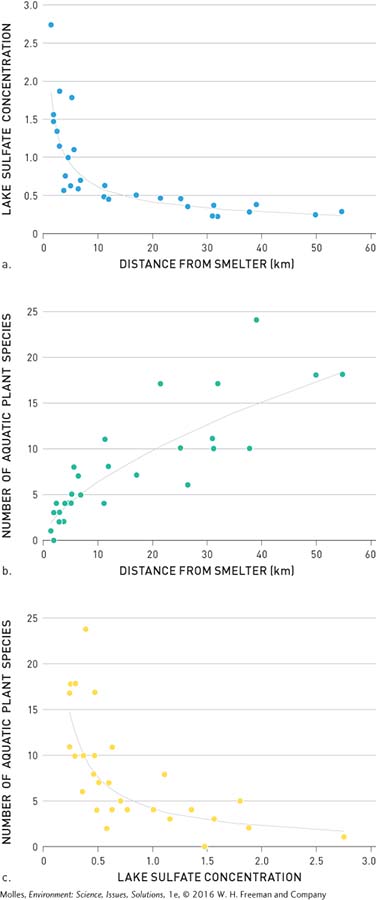
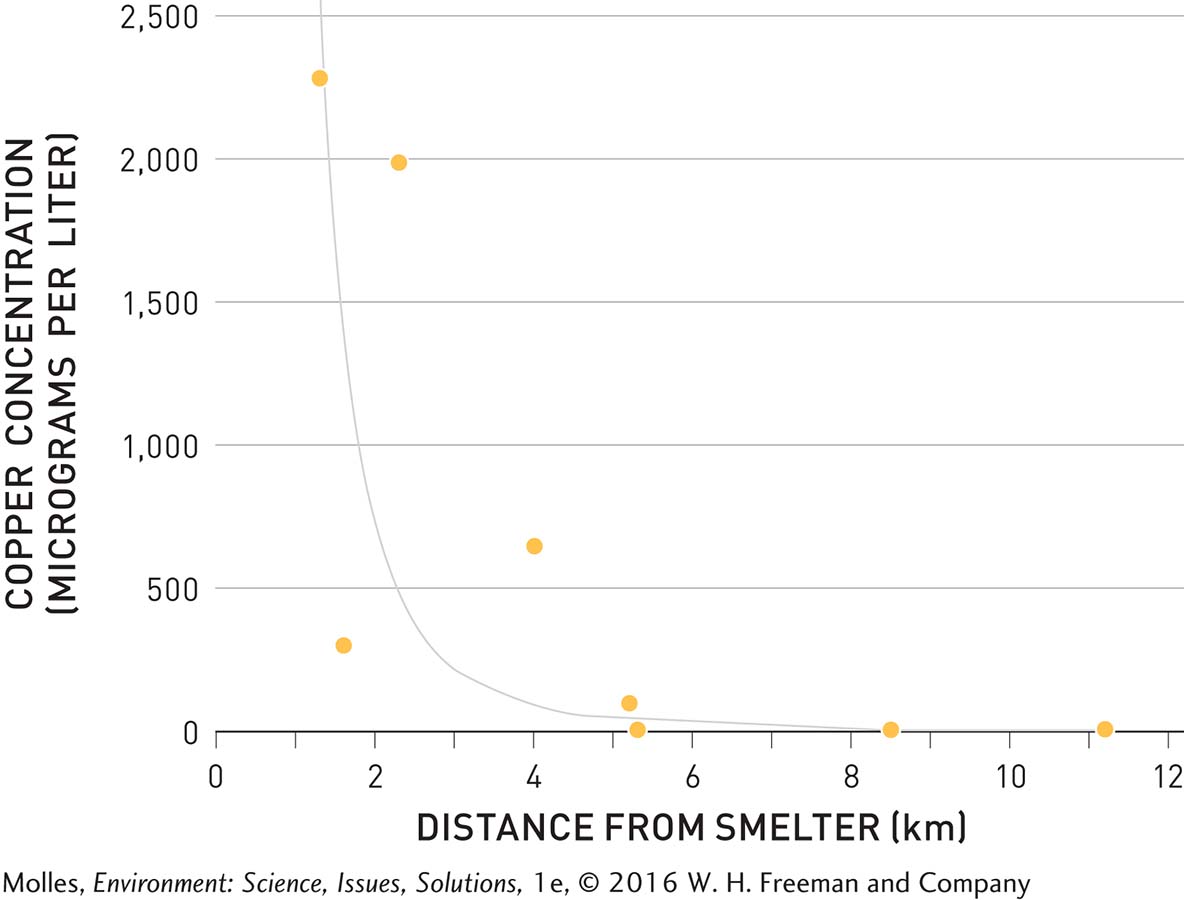
You might be wondering whether acid rain impacted the many lakes around Sudbury, Ontario. Gorham and Gordon also surveyed the aquatic plants living in study lakes near Sudbury. They found that the sulfate concentrations in the lakes decreased with distance from metal smelters and that the number of aquatic plant species increased with distance from smelters. By plotting sulfate concentration against number of aquatic plant species in these lakes, they determined that aquatic plant species numbers decline as sulfate concentration increases (Figure 13.20). The concentrations of sulfate in these lakes, however, didn’t seem high enough to kill the plants, so Gorham and Gordon wondered whether another pollutant was responsible. Indeed, they found that copper, a metal toxic to plants at higher concentrations, was highest in snow near the smelters and declined with distance (Figure 13.21).
Think About It
How would you explain the fact that the earliest discoveries of acid deposition impacts were in terrestrial ecosystems, whereas the phenomenon of cultural eutrophication was first documented in aquatic ecosystems?
Page 404What processes connect the impacts of acid rain in terrestrial ecosystems to impacts on aquatic ecosystems?
What are the mechanisms by which acid rain impacts ecosystems?