9.1–9.3 Science

Think about the last time there was a power outage in your city. If it lasted just a few hours, it was likely a diversion as you sat around and shared stories with your family in the dark as the ice cream in your freezer melted. Now imagine if the whole world went dark and the global supply of fuel was exhausted. Airports would shut down. Factories would close their doors. The food supply would diminish. Your only source of news would be the neighbors. Life as we know it would come to a halt. It’s not such a far-
9.1 Fossil fuels provide energy in chemical form
fossil fuels Fossilized organic material, mainly the remains of ancient photosynthetic organisms that converted the Sun’s radiant energy into chemical energy (e.g., coal, oil, natural gas).
Coal, oil, and natural gas are called fossil fuels because they are the fossilized remains of ancient photosynthetic organisms that converted the radiant energy in sunlight to chemical energy. That energy is stored in the form of molecular chains made up of hydrogen and carbon. Burning these fuels breaks up those molecular chains and liberates smaller molecules, including water and carbon dioxide, along with pollutants and ash particles known as black carbon. By burning them, the stored energy is released, primarily in the form of heat.
Coal
coal Sedimentary or metamorphic rock high in carbon and energy content formed over millions of years under conditions of high pressure and temperature (lignite, sub-
As the leaves, branches, and trunks of ancient plants accumulated in freshwater swamps some 100 to 300 million years ago, they formed organic-
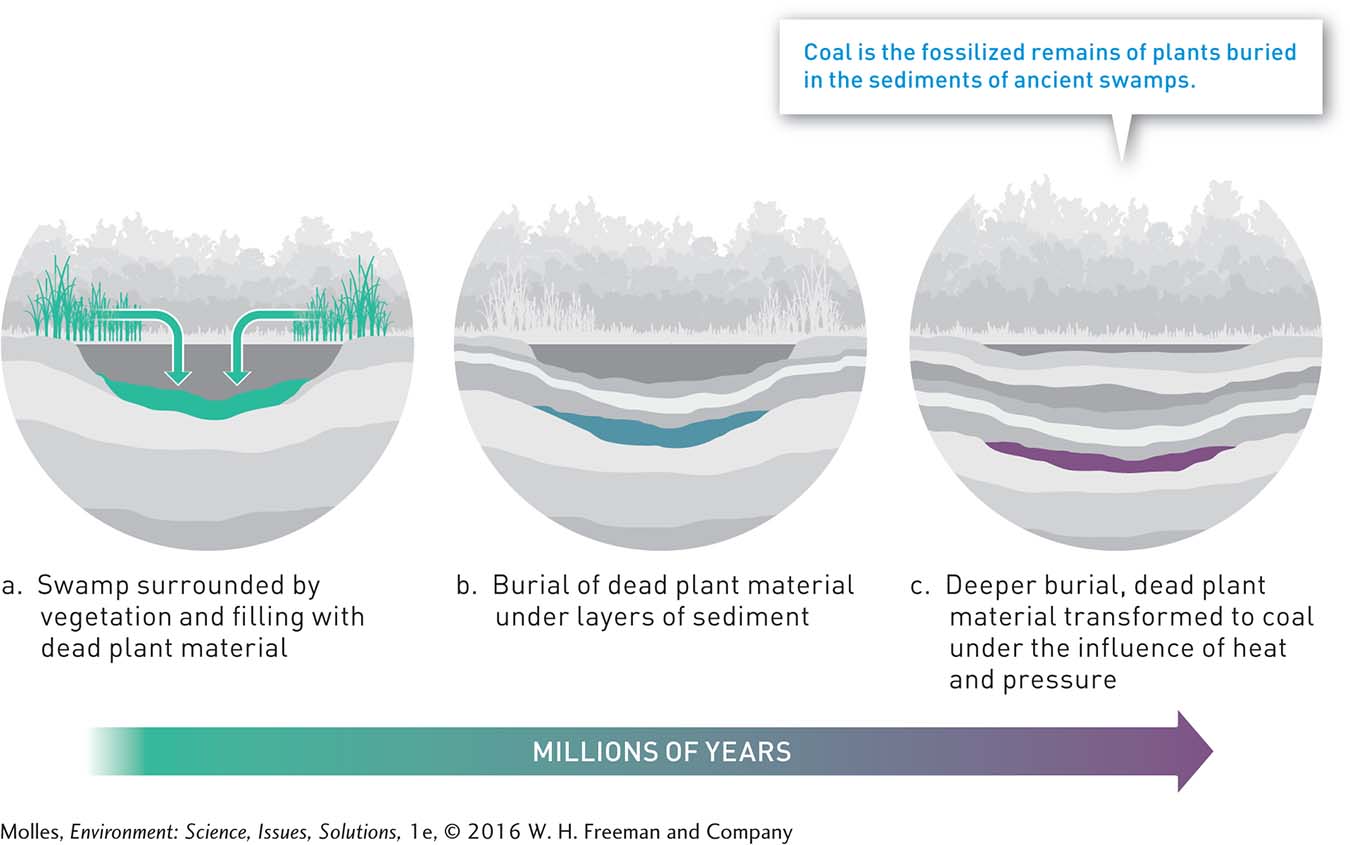
Geologists classify coal into four major grades, which differ in carbon and energy content (Figure 9.2). The differences among coal grades are mainly the result of variations in their age and the amount of heat and pressure to which they were exposed during development. Lignite, the youngest of the coal grades with the lowest carbon and energy content, was subjected to less heat and pressure during development than were the other grades. The other coal grades, listed in order of increasing carbon content, are sub-
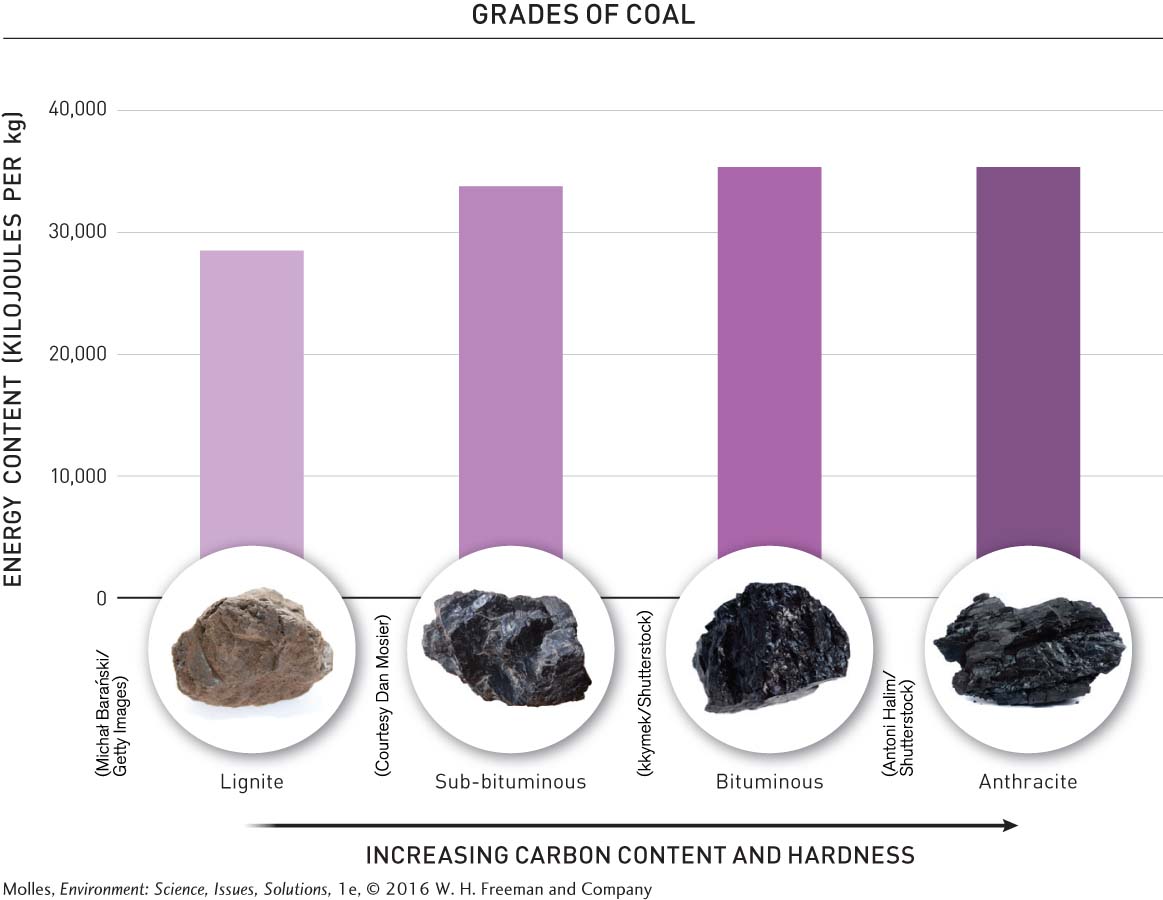
Humans have used coal as a source of heating since prehistoric times. Native Americans once burned it in their pottery kilns, and the steamships and railroads of the Industrial Revolution were powered by coal boilers. Today, coal is most commonly used to generate electricity. For example, more than 90% of coal mined in the United States is used in coal-
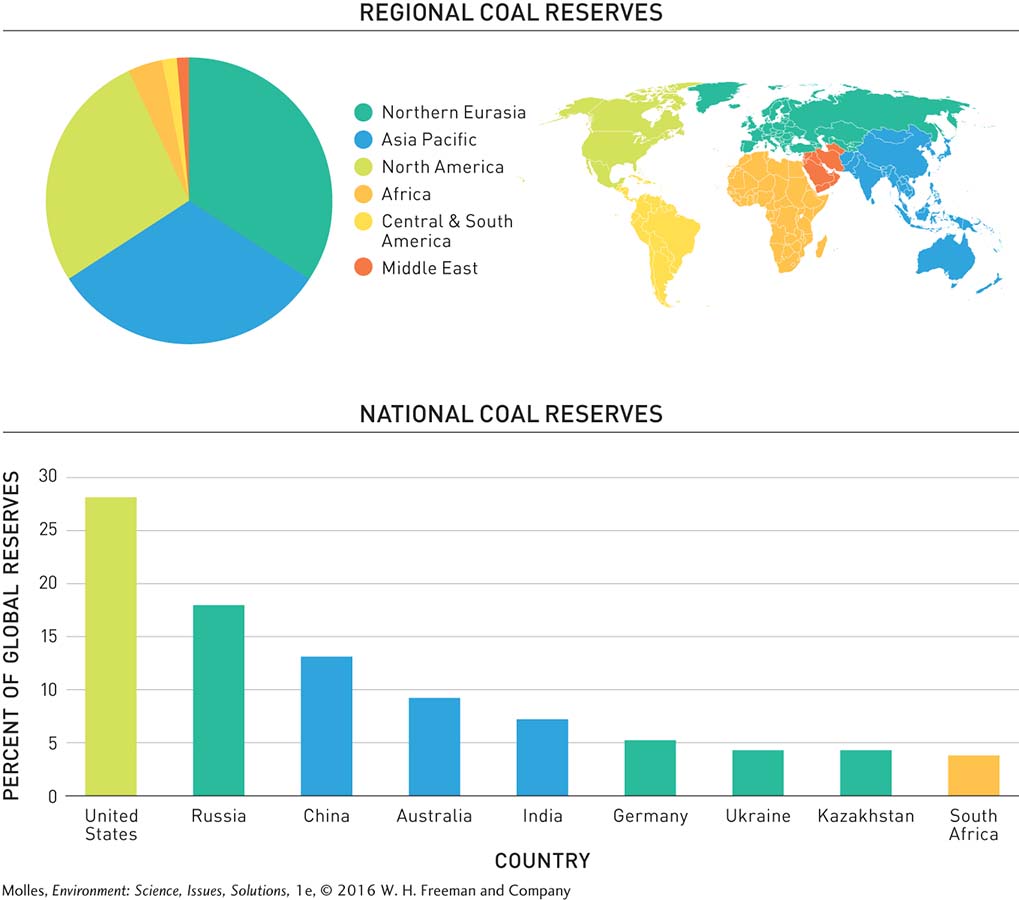
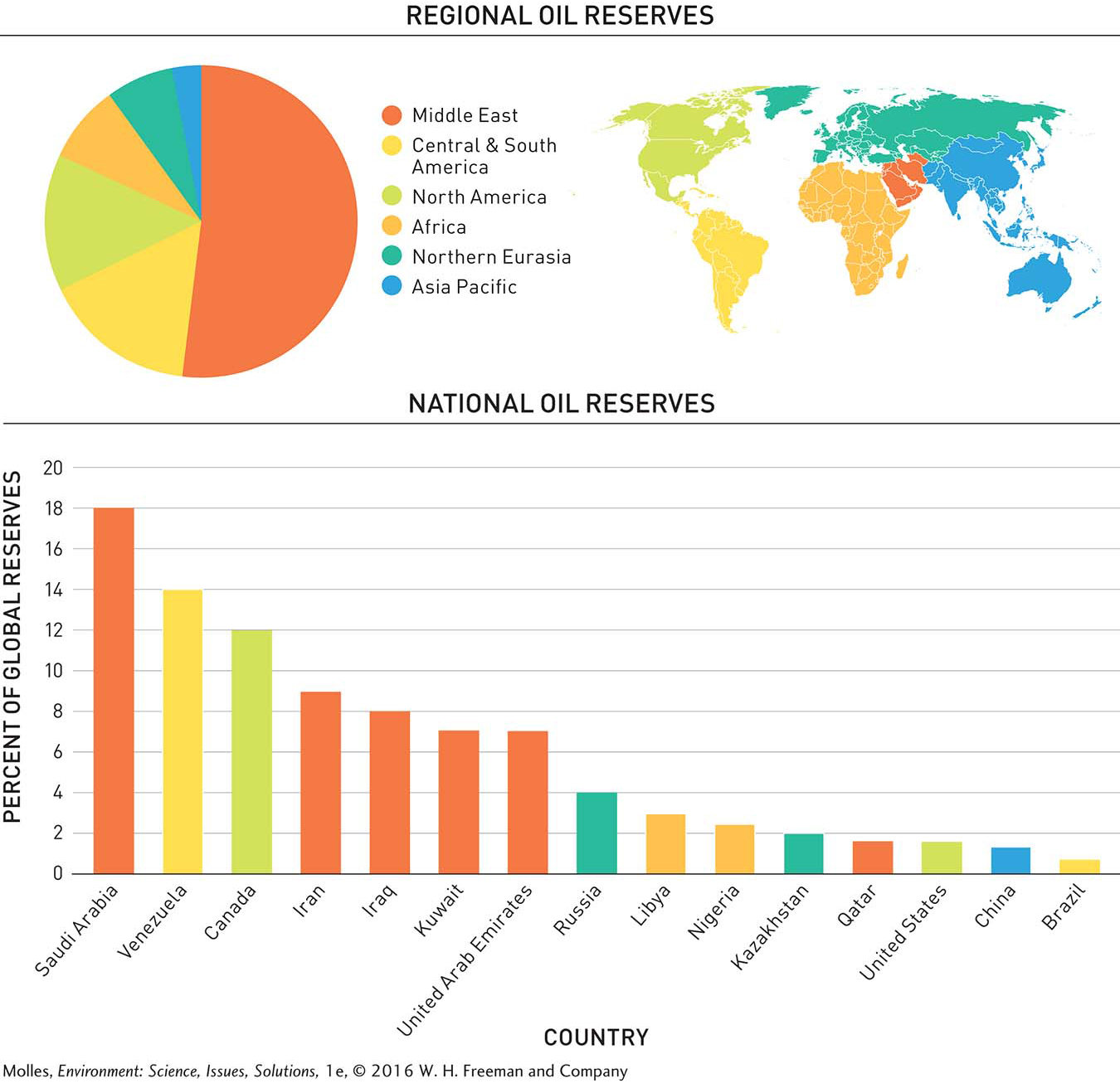
In 2008 energy experts estimated that about 93% of the world’s known coal reserves occur in three regions of the world: Northern Eurasia, Asia Pacific, and North America. Meanwhile, Africa, South and Central America, and the Middle East collectively are estimated to hold just 7% of world reserves. Closer analysis shows that proven coal reserves are even more restricted geographically. Just nine nations harbor more than 90% of the world’s coal reserves (Figure 9.3). At 28% of the world total, the coal reserves in the United States exceed all others.
Petroleum
petroleum (crude oil) A mixture of hydrocarbons contained in sedimentary rocks of marine origin; developed from the accumulated remains of algae and zooplankton deposited on the sea floor over millions of years.
kerogen A waxy substance found in shale and other sedimentary rocks that yields oil when heated; occurs during an intermediate stage of petroleum formation.
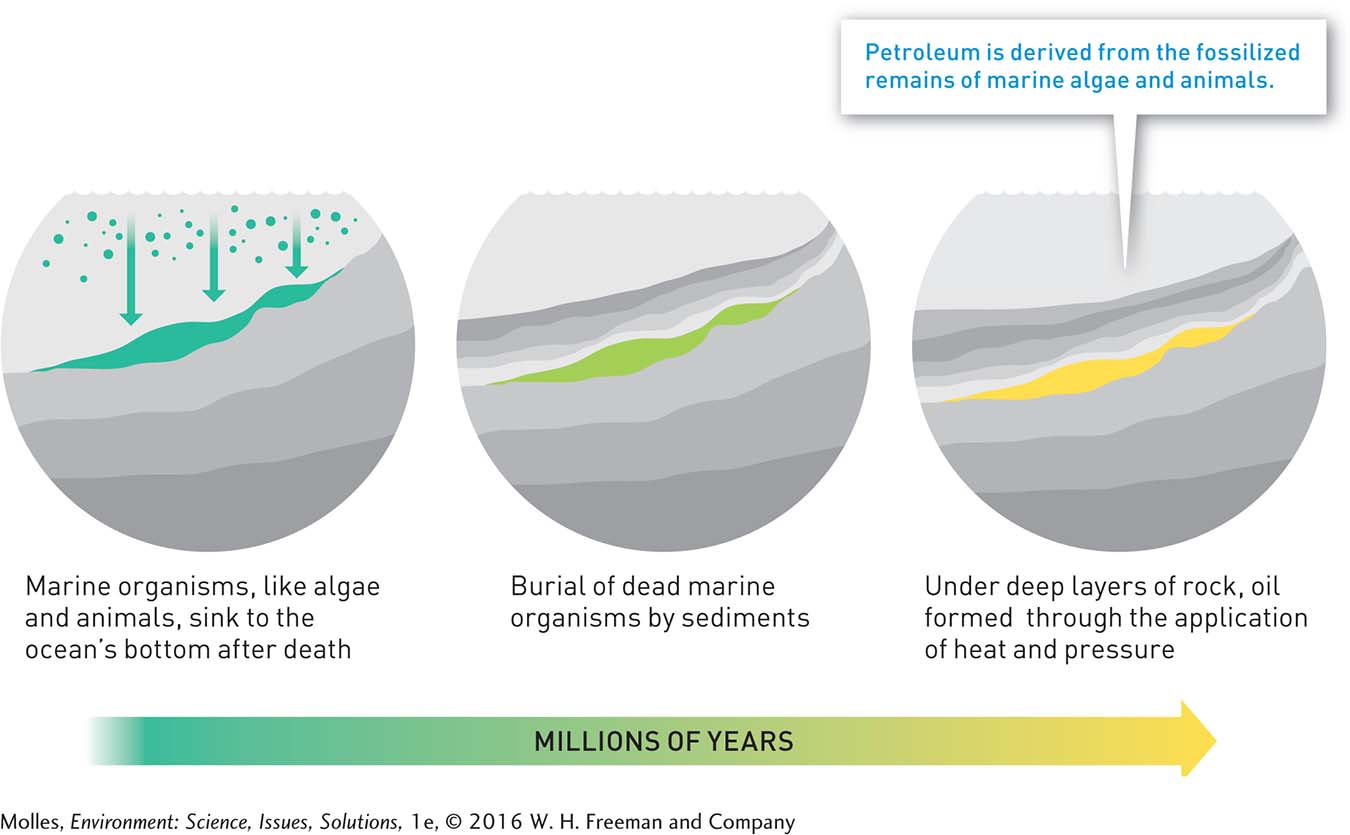
Petroleum, or crude oil, formed in the oceans from the accumulated remains of algae and zooplankton deposited on the sea floor over millions of years (Figure 9.4). These deposits mixed with sands and silt, and eventually this organic-

What do the rich oil fields in places like Texas and North Dakota suggest about the geologic history of these regions?
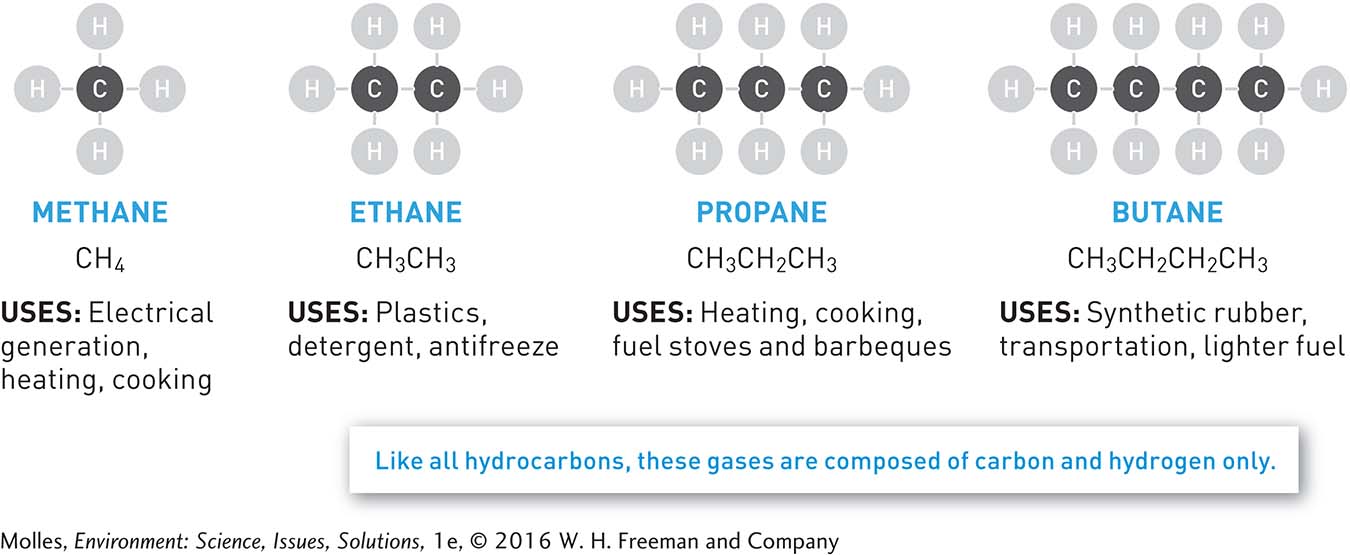
hydrocarbon An organic molecule made up of carbon and hydrogen only; the simplest hydrocarbon is methane (CH4), the main component of natural gas.
Chemically speaking, crude oil is a mixture of hydrocarbons, molecular chains consisting of only carbon and hydrogen. The smallest hydrocarbon, made up of four hydrogen atoms bonded to a single carbon atom, is methane (CH4). To be useful and safe, crude oil must be refined, a process that involves separating the hydrocarbons into fractions containing hydrocarbons with the same number of carbon atoms.
The main principle behind the refining of oil is that different sizes of hydrocarbons have different molecular weights and will boil (or condense) at different temperatures. To separate these hydrocarbons, heated crude is pumped into the refinery column, as shown in Figure 9.5. Because the temperature within the column decreases from bottom to top, the heaviest hydrocarbons (e.g., heating oil) condense and flow out in the lower layers. Meanwhile, the lightest hydrocarbons (e.g., methane) rise to the very top of the column, where they are collected. These gases are typically pressurized until they turn to liquid, resulting in liquefied petroleum gas, or LPG.
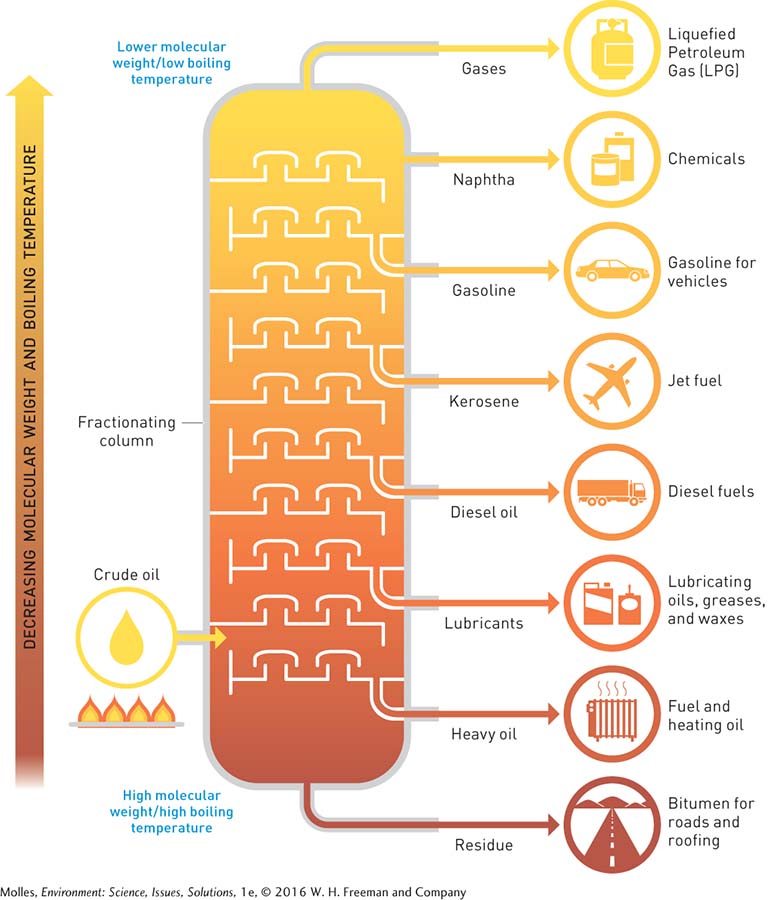
Petroleum ends up in our cars and trucks in its most familiar forms as gasoline (petrol) and diesel fuel. However, various oil products are in high demand to lubricate engines, tar our roads, or heat our homes (Figure 9.5). For many of these applications, alternatives do not work as well or are currently more costly.
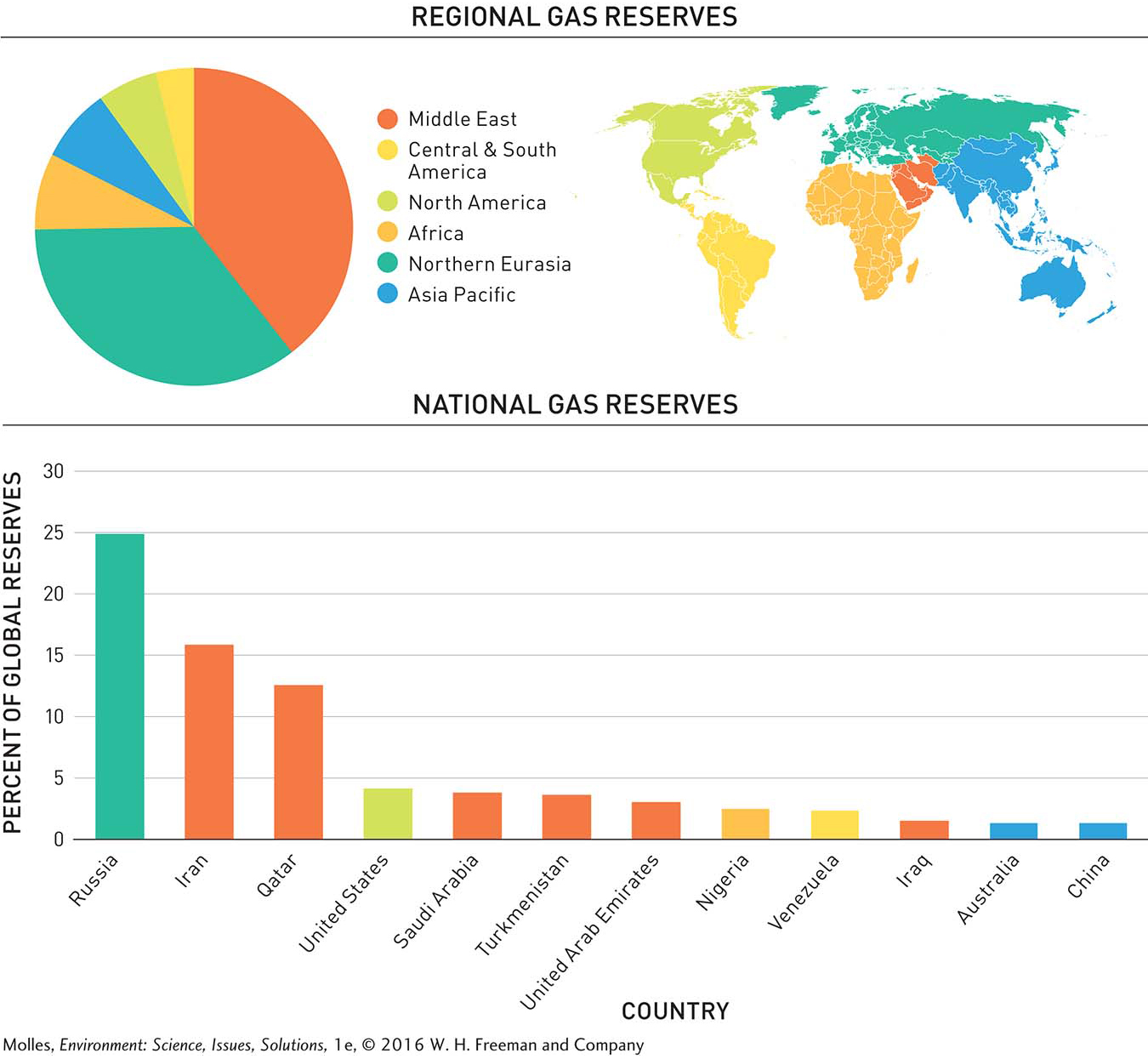
The Middle East has oil reserves that account for over half of the world total and dwarf those of all other regions. After the Middle East, South and Central America, North America, Africa, and Northern Eurasia, each control from approximately 7% to 16% of global reserves. Within the world’s regions, just 15 countries control more than 90% of the world’s known oil reserves (Figure 9.6).
Natural Gas
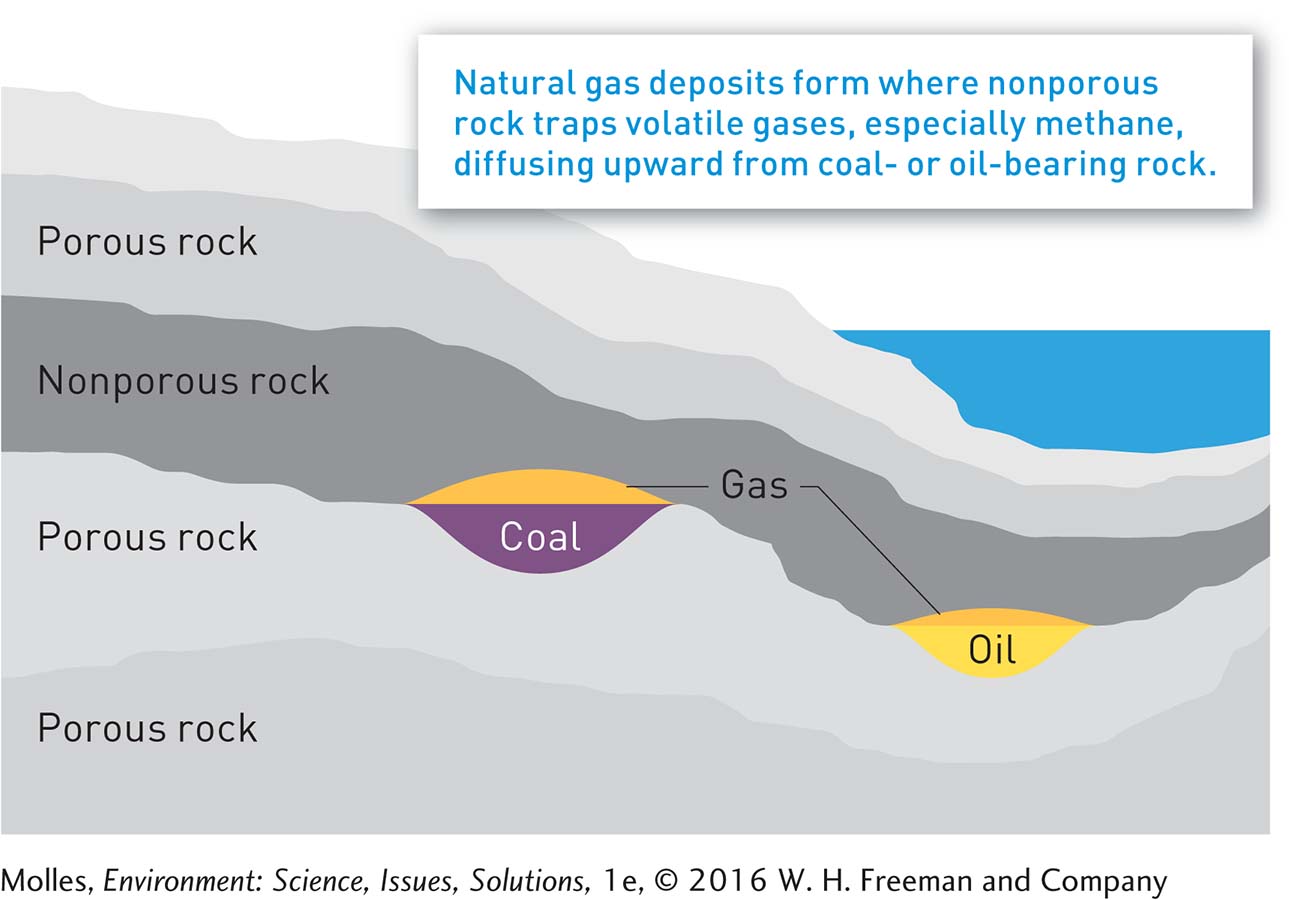
Natural gas is a mixture of gaseous hydrocarbons—

What does the shift in the view of natural gas from “waste product” to valuable resource suggest about changes in energy supply and demand over time?
Natural gas associated with oil deposits and coal beds was once considered a hazard, because it could explode during drilling, and a waste product, because it was difficult to store and transport. Now, however, natural gas is used widely in residential, commercial, and industrial settings (Figure 9.9), where it accounts for about one-

Think About It
The same processes that produced fossil fuels, such as coal and petroleum, are still occurring on Earth today. Why, then, are fossil fuels considered “nonrenewable”?
Explain why the author Thom Hartmann was correct when he referred to fossil fuels as “ancient sunlight.”