HOW DO WE KNOW?
FIG. 8.12
Do chlorophyll molecules operate on their own or in groups?
BACKGROUND By about 1915, scientists knew that chlorophyll was the pigment responsible for absorbing light energy in photosynthesis. However, it was unclear how these pigments contributed to the reduction of CO2. The American physiologists Robert Emerson and William Arnold set out to determine the nature of the “photochemical unit” by quantifying how many chlorophyll molecules were needed to produce one molecule of O2.
EXPERIMENT Emerson and Arnold exposed flasks of the green alga Chlorella to flashes of light of such short duration (10−5 s) and high intensity (several times that of full sunlight) that each chlorophyll molecule would be “excited” only once. They also made sure that the time between flashes was long enough to allow the reactions resulting from each flash to run to completion. They then measured O2 production per flash of high intensity light, and determined the concentration of chlorophyll present in their solution of cells. Finally, they divided the number of chlorophyll molecules per mm3 of solution by O2 production per flash per mm3 of cells in solution.
RESULTS
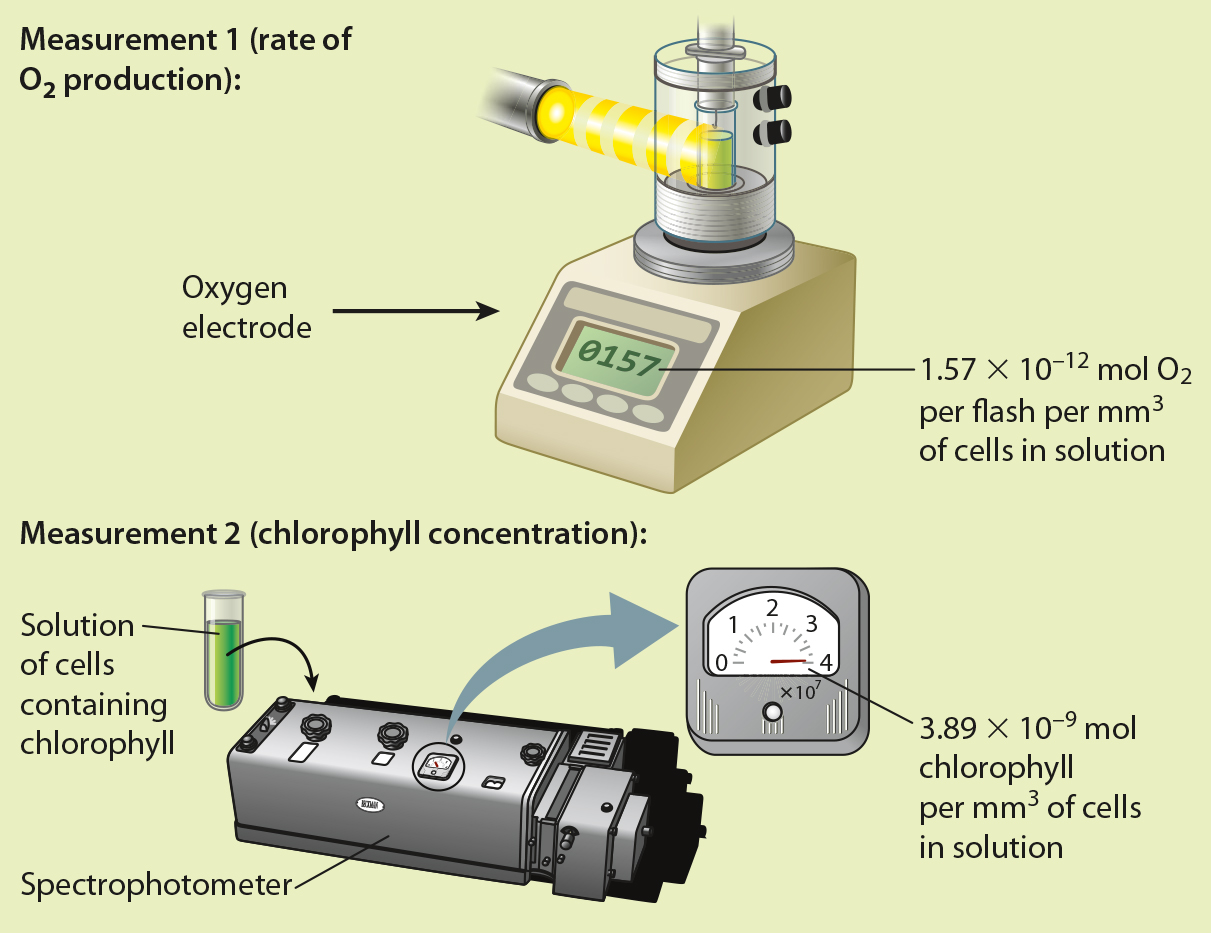
CONCLUSION Because the amount of chlorophyll in their flask was much greater than O2 production per flash, Emerson and Arnold concluded that each photochemical unit contains many chlorophyll molecules.
FOLLOW-
SOURCE Emerson, R., and W. Arnold. 1932. “The Photochemical Reaction in Photosynthesis.” Journal of General Physiology 16:191–