Cell adhesion molecules allow cells to attach to other cells and to the extracellular matrix.
In 1907, American embryologist H. V. Wilson discovered that if he pressed a live sponge through fine cloth he could break up the sponge into individual cells. Then if he swirled the cells together, they would coalesce back into a group resembling a sponge. If he swirled the cells from sponges of two different species together, he observed that the cells sorted themselves out—
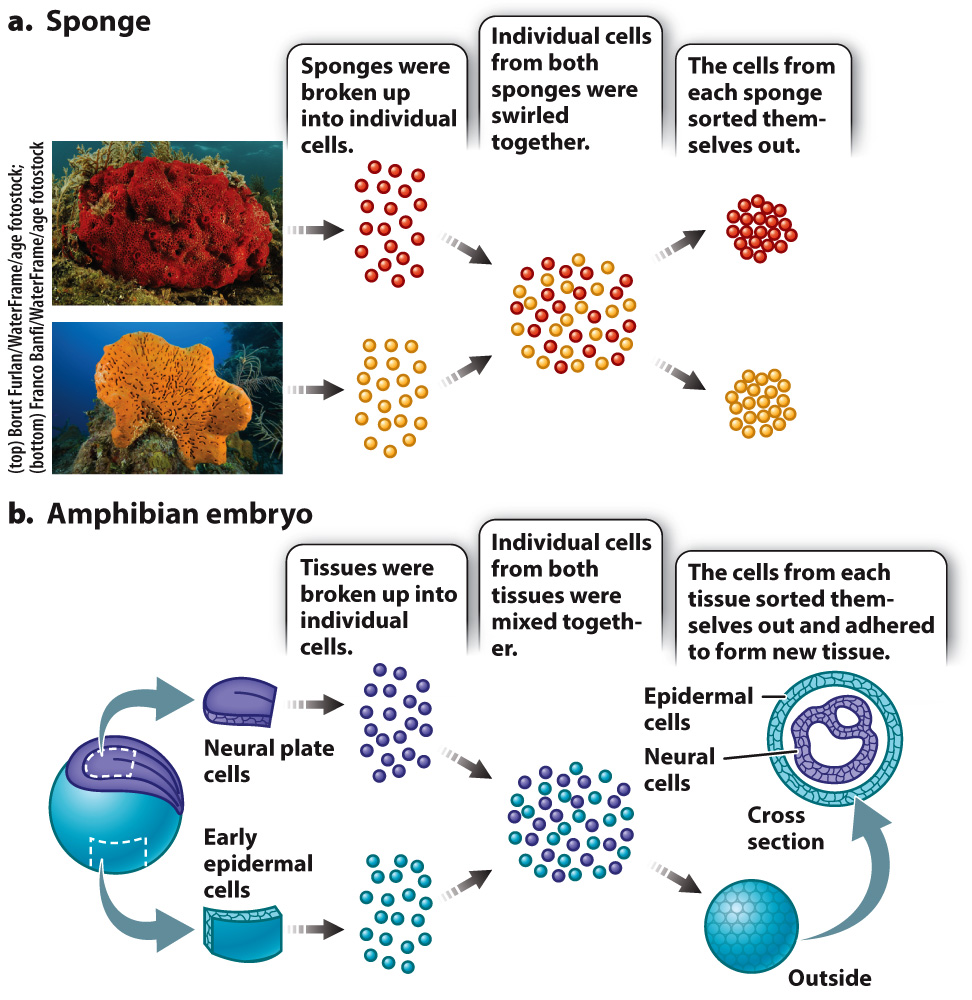
Cells are able to sort themselves because of the presence of various proteins on their surface called cell adhesion molecules that attach cells to one another or to the extracellular matrix. While a number of cell adhesion molecules are now known, the cadherins (calcium-
Cadherins are transmembrane proteins (Chapter 5). The extracellular domain of a cadherin molecule binds to the extracellular domain of a cadherin of the same type on an adjacent cell. The cytoplasmic portion of the protein is linked to the cytoskeleton, including microfilaments and intermediate filaments (Fig. 10.13a). This arrangement provides structural continuity from the cytoskeleton of one cell to the cytoskeleton of another, increasing the strength of tissues and organs.
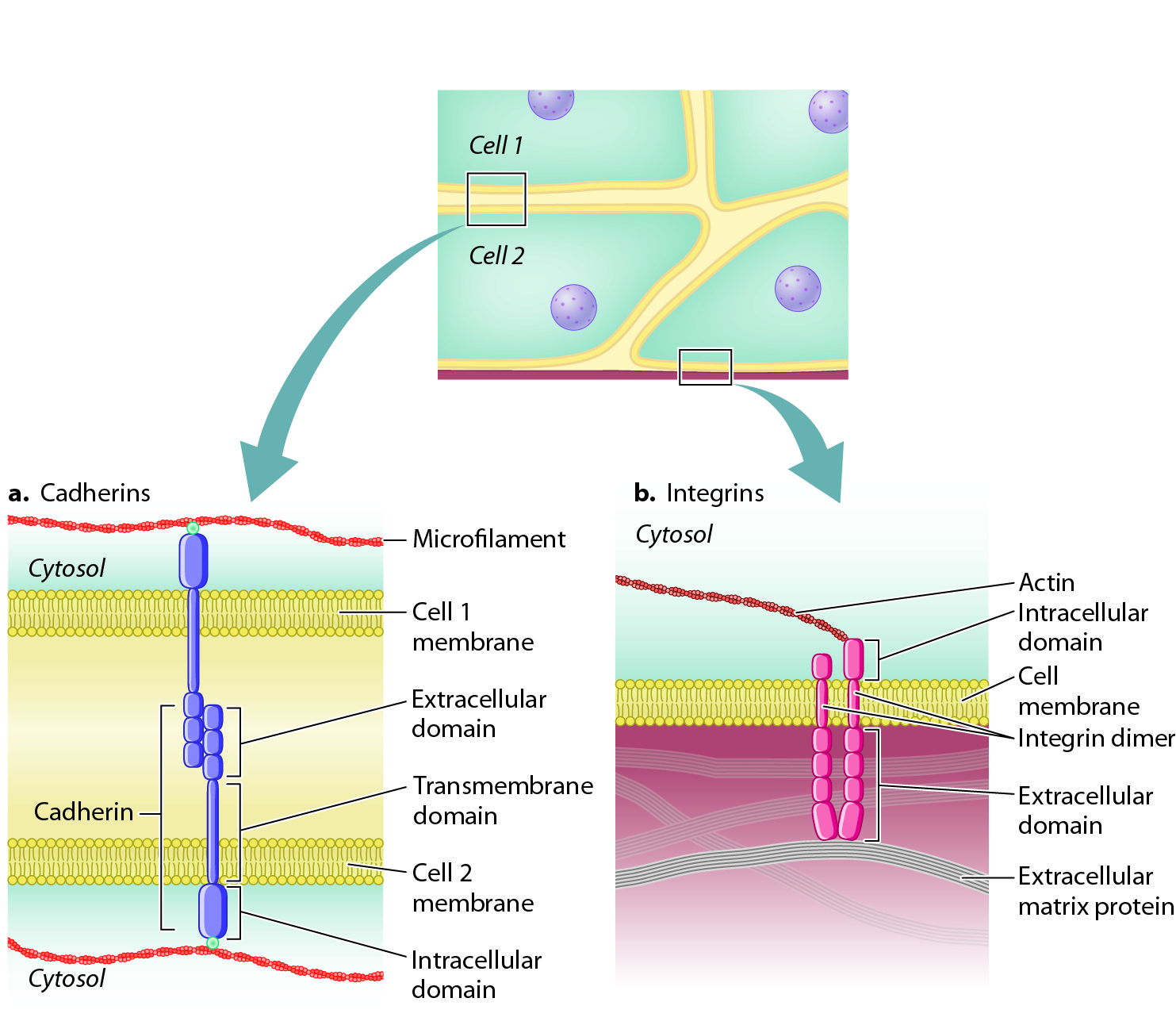
As well as being stably connected to other cells, cells also attach to proteins of the extracellular matrix. Cell adhesion molecules that enable cells to adhere to the extracellular matrix are called integrins. Like cadherins, integrins are transmembrane proteins, and their cytoplasmic domain is linked to microfilaments or intermediate filaments (Fig. 10.13b). Also like the cadherins, integrins are of many different types, each binding to a specific extracellular matrix protein. Integrins are present on the surface of virtually every animal cell. In addition to their role in adhesion, integrins also act as receptors that communicate information about the extracellular matrix to the interior of the cell (section 10.4)