The extracellular matrix is abundant in connective tissues of animals.
The extracellular matrix of animals, like that of plants, is a mixture of proteins and polysaccharides secreted by cells. The animal extracellular matrix is composed of large fibrous proteins, including collagen, elastin, and laminin, which impart tremendous tensile strength. These fibrous proteins are embedded in a gel-
The extracellular matrix can be found in abundance in animal connective tissue (Fig. 10.16). Connective tissue structurally integrates and supports various parts of the body and, in this way, is necessary for multicellularity. All animals express similar connective tissue proteins, highlighting their importance and evolutionarily conserved function. Connective tissue underlies all epithelial tissues, as we have seen (section 10.1). For example, the dermis of the skin is connective tissue. It provides support and nutrients to the overlying epidermis. The main type of cell in the dermis is the fibroblast. Fibroblasts synthesize most of the extracellular matrix proteins.
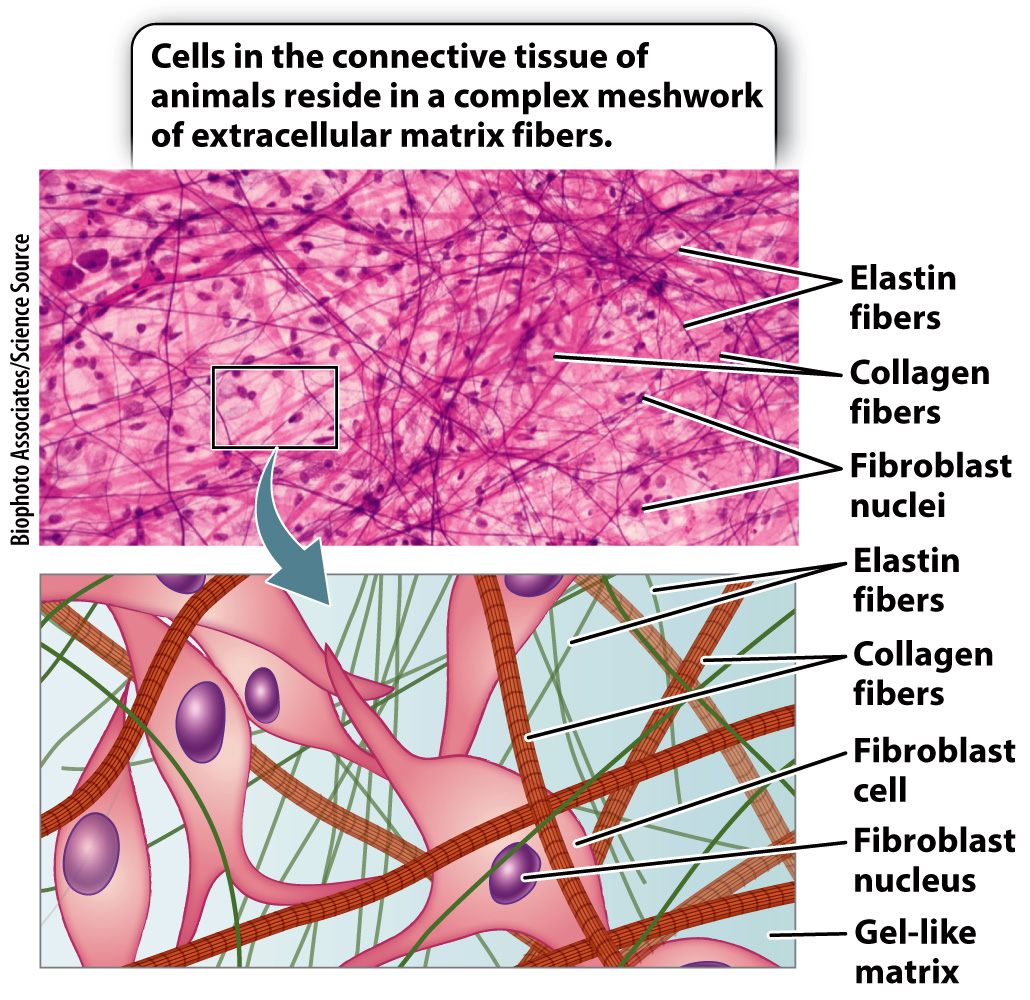
Connective tissue is unusual compared to other tissue types in that it is dominated by the extracellular matrix and has a low cell density. Consequently, the extracellular matrix determines the properties of different types of connective tissue. Other more specialized types of animal connective tissue include bone, cartilage, and tendon.
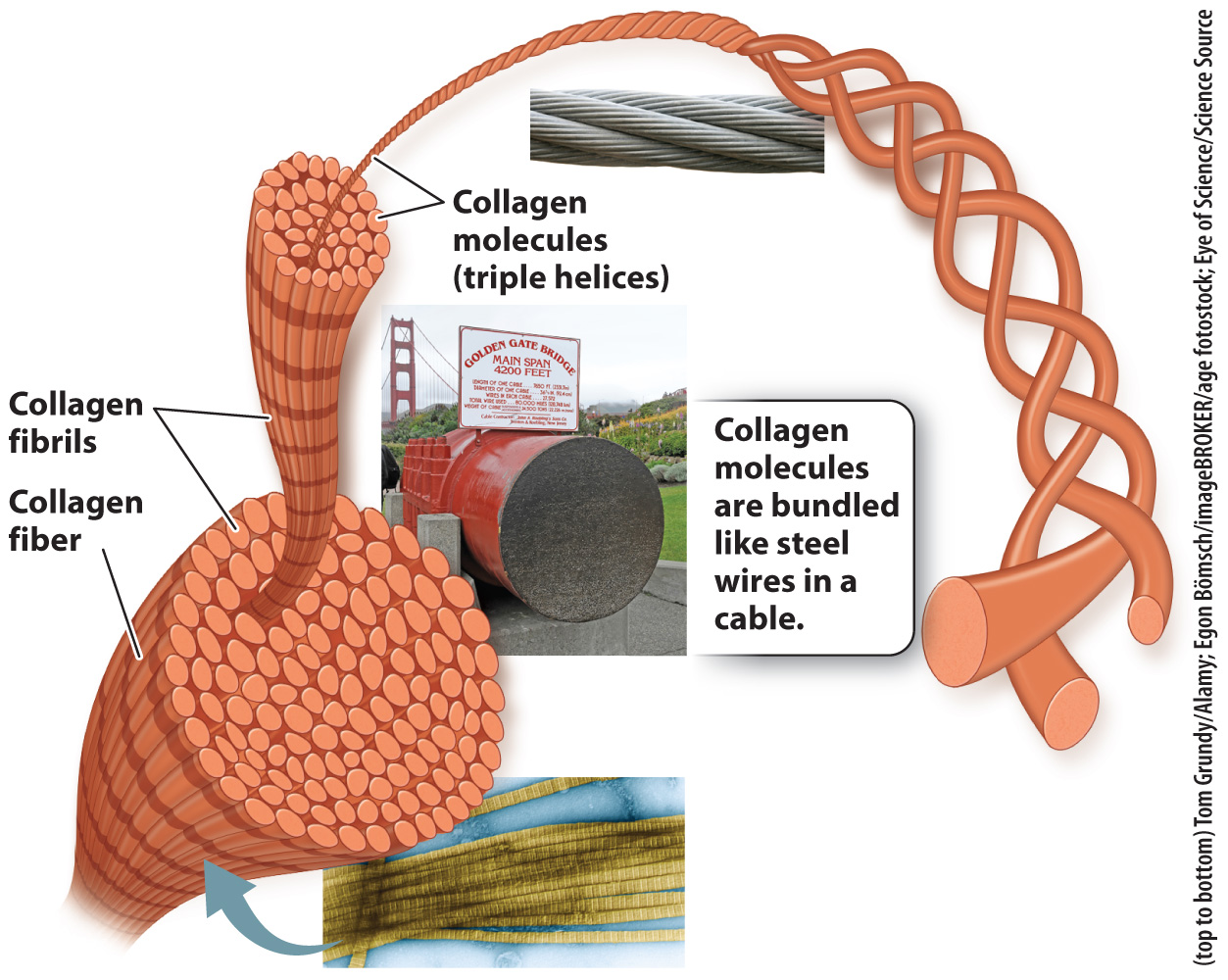
Collagen is the most abundant protein in the extracellular matrix of animals. There are more than 20 different forms of collagen, and in humans collagen accounts for almost a quarter of the protein present in the body. Over 90% of this collagen is type I collagen, which is present in the dermis of your skin, where it provides strong, durable support for the overlying epidermis. The tendons that connect your muscles to bones and the ligaments that connect your bones to other bones are able to withstand the physical stress placed on them because they are made up primarily of collagen.
Collagen’s strength is related to its structure. Like a rope or a cable, collagen is composed of intertwined fibers that make it much stronger than if it were a single fiber of the same diameter. A collagen molecule consists of three polypeptides wound around one another in a triple helix. A bundle of collagen molecules forms a fibril, and the fibrils are assembled into fibers (Fig. 10.17). Once multiple collagen fibers are assembled into a ligament or tendon, the final structure is incredibly strong.
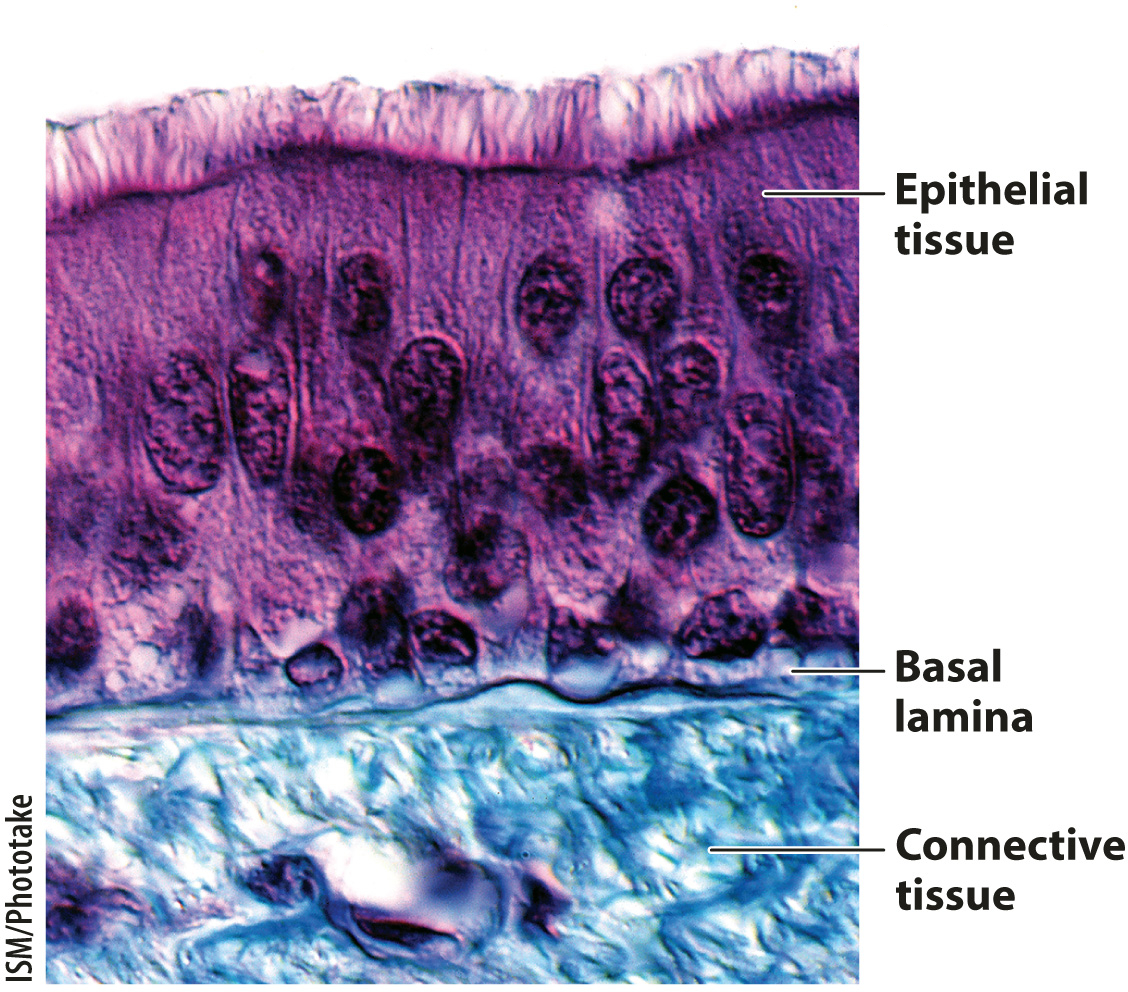
The basal lamina is a specialized layer of extracellular matrix that is present beneath all epithelial tissues, including the lining of the digestive tract, epidermis of the skin, and endothelial cells that line the blood vessels of vertebrates (Fig. 10.18). The role of the basal lamina is to provide a structural foundation for these epithelial tissues. The basal lamina is made of several proteins, including a special type of collagen. The triple-