DNA editing can be used to alter gene sequences almost at will.
Recombinant DNA technology combines existing DNA from two or more different sources. Its usefulness in research, medicine, and agriculture, however, is limited by the fact that it can make use only of existing DNA sequences. Therefore, scientists have also developed many different techniques to alter the nucleotide sequence of almost any gene in a deliberate, targeted fashion. In essence, these techniques allow researchers to “rewrite” the nucleotide sequence so that specific mutations can be introduced into genes to better understand their function, or mutant versions of genes can be corrected to restore normal function. Collectively, these techniques are known as DNA editing.
One of the newest and most exciting ways to edit DNA goes by the acronym CRISPR (clustered regularly interspaced short palindromic repeats), and it was discovered in an unexpected way. Researchers noted that about half of all species of bacteria and most species of Archaea contain small segments of DNA of about 20–50 base pairs derived from plasmids or viruses, but their function was at first a mystery. Later, it was discovered that they play a role in the bacterial immune system. When a bacterium is infected by a virus for the first time, it makes a copy of part of the viral genome and incorporates it into its genome. On subsequent infection by the same virus, the DNA copy of the viral genome is transcribed to RNA that combines with a protein that has a DNA-cleaving function. The RNA serves as a guide to identify target DNA in the virus by complementary base pairing, and the protein cleaves the target DNA. In this way, bacteria “remember” and defend themselves from past infections. The phrase “clustered regularly interspaced short palindromic repeats” describes the organization of the viral DNA segments in the bacterial genome.
In modern genetic engineering, the CRISPR mechanism is put to practical use to alter the nucleotide sequence of almost any gene in any kind of cell. One method is outlined in Fig. 12.21. The first step is to transform a cell with a plasmid containing sequences that code for a CRISPR RNA as well as the CRISPR-associated protein Cas9. The RNA contains a region that can form a hairpin-shaped structure, as well as a region engineered to have bases complementary to any DNA molecule in the cell to be altered, known as the target DNA (Fig. 12.21a). When the RNA undergoes base pairing with the target DNA, Cas9 cleaves the target DNA (Fig. 12.21b). Exonucleases in the cell then expand the gap (Fig. 12.21c). The gap can be repaired using another DNA molecule that serves as a template for editing the target DNA (Fig. 12.21d). This editing template DNA is introduced to the cell by a plasmid and contains a sequence of interest to replace the degraded sequence of the target DNA, flanked by sequences complementary to the target. The strands of the gapped target DNA undergo base pairing with the complementary ends of the editing template, and DNA synthesis elongates the target DNA strands and closes the gap (Fig. 12.21d). The result is that the target DNA is restored, but its sequence has been altered according to the sequence present in the editing template (Fig. 12.21e).
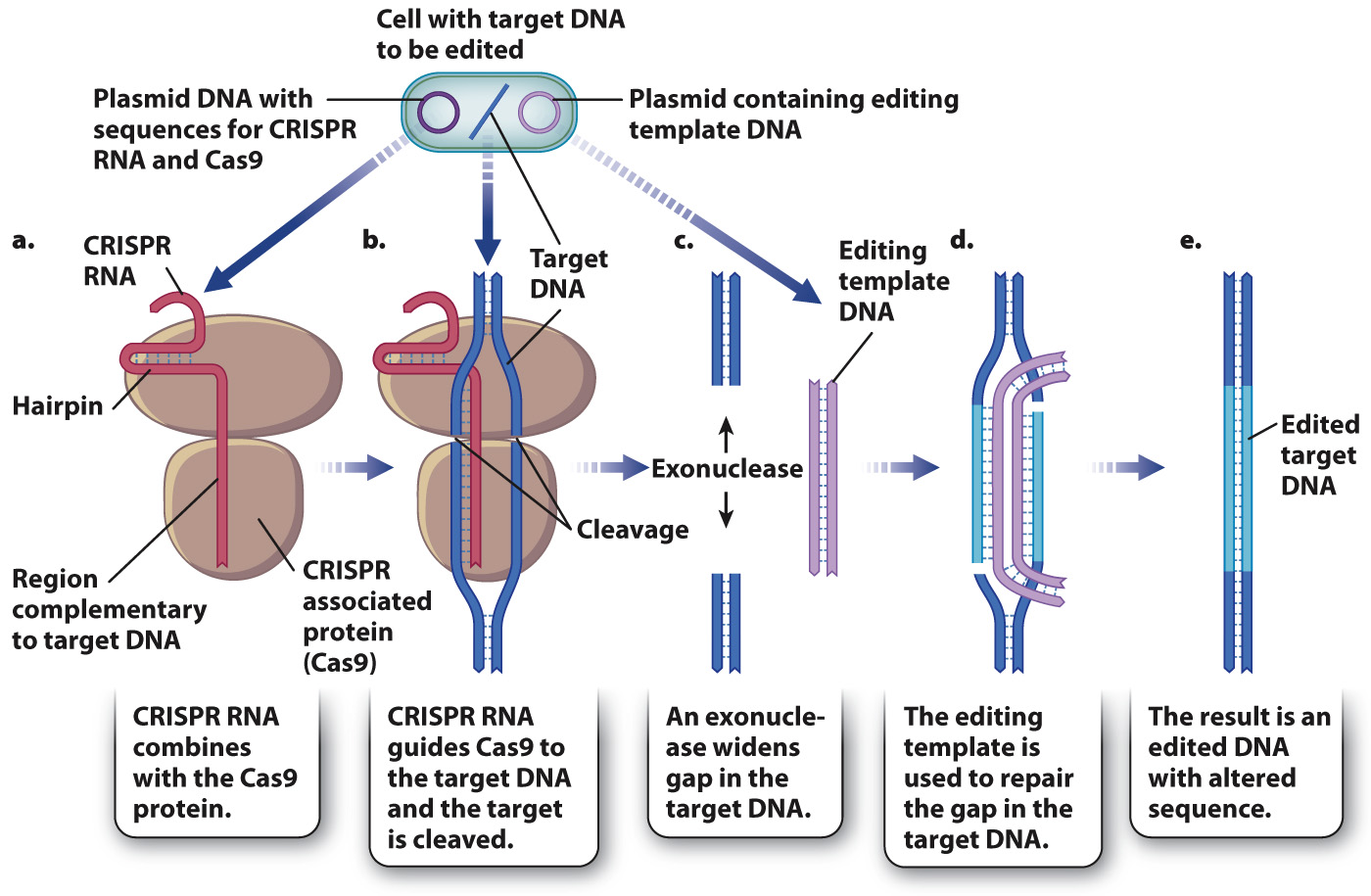
FIG. 12.21 DNA editing. In this example, CRISPR RNA and its associated protein are used to cleave target DNA, and double-stranded template DNA is used in DNA repair to alter its sequence.
Page 268
DNA editing by CRISPR is technically straightforward and highly efficient. The method has generated great interest because of its potential to correct genetic disorders of the blood, immune system, or other tissues and organs in which only a subset of cells with restored function can alleviate symptoms.
