Lactose utilization in E. coli is the pioneering example of transcriptional regulation.
The principle that the product of one gene can regulate transcription of other genes was first discovered in the 1960s by François Jacob and Jacques Monod, who studied how the bacterium E. coli regulates production of the proteins needed for utilization of the sugar lactose. Lactose consists of one molecule each of the sugars glucose and galactose covalently linked by a β-(beta-
Jacob and Monod began their research with the observation that active β-galactosidase enzyme is observed in cells only in the presence of lactose or certain molecules chemically similar to lactose. Why is this so? Fig. 19.14 describes and tests two hypotheses. One is that lactose stabilizes an unstable form of β-galactosidase produced by all cells all the time. The other is that lactose leads to the expression of the gene for β-galactosidase. The experiment shown in Fig. 19.14 demonstrated that lactose turns on the β-galactosidase gene and does not stabilize or activate the enzyme encoded by the gene. How lactose activates expression of the β-galactosidase gene was the subject of additional experiments. These Nobel Prize–
HOW DO WE KNOW?
FIG. 19.14
How does lactose lead to the production of active β-galactosidase enzyme?
BACKGROUND Active β-galactosidase enzyme is observed only in E. coli cells that are growing in the presence of lactose.
HYPOTHESIS One hypothesis is that the enzyme is always being produced, but is produced in an unstable form that breaks down rapidly in the absence of lactose. A second hypothesis is that the enzyme is stable, but is produced only in the presence of lactose.
EXPERIMENT François Jacob and Jacques Monod exposed a culture of growing cells to lactose and later removed it. They measured the amount of β-galactosidase present in the culture during the experiment.
RESULTS Almost immediately upon addition of lactose, β-galactosidase began to accumulate, and its amount steadily increased. When lactose was removed, the enzyme did not disappear immediately (as would be the case if it were unstable). Instead, the amount of enzyme remained the same as when lactose was present. This result is expected only if β-galactosidase is a stable enzyme that is synthesized when lactose is added and stops being synthesized when lactose is removed.
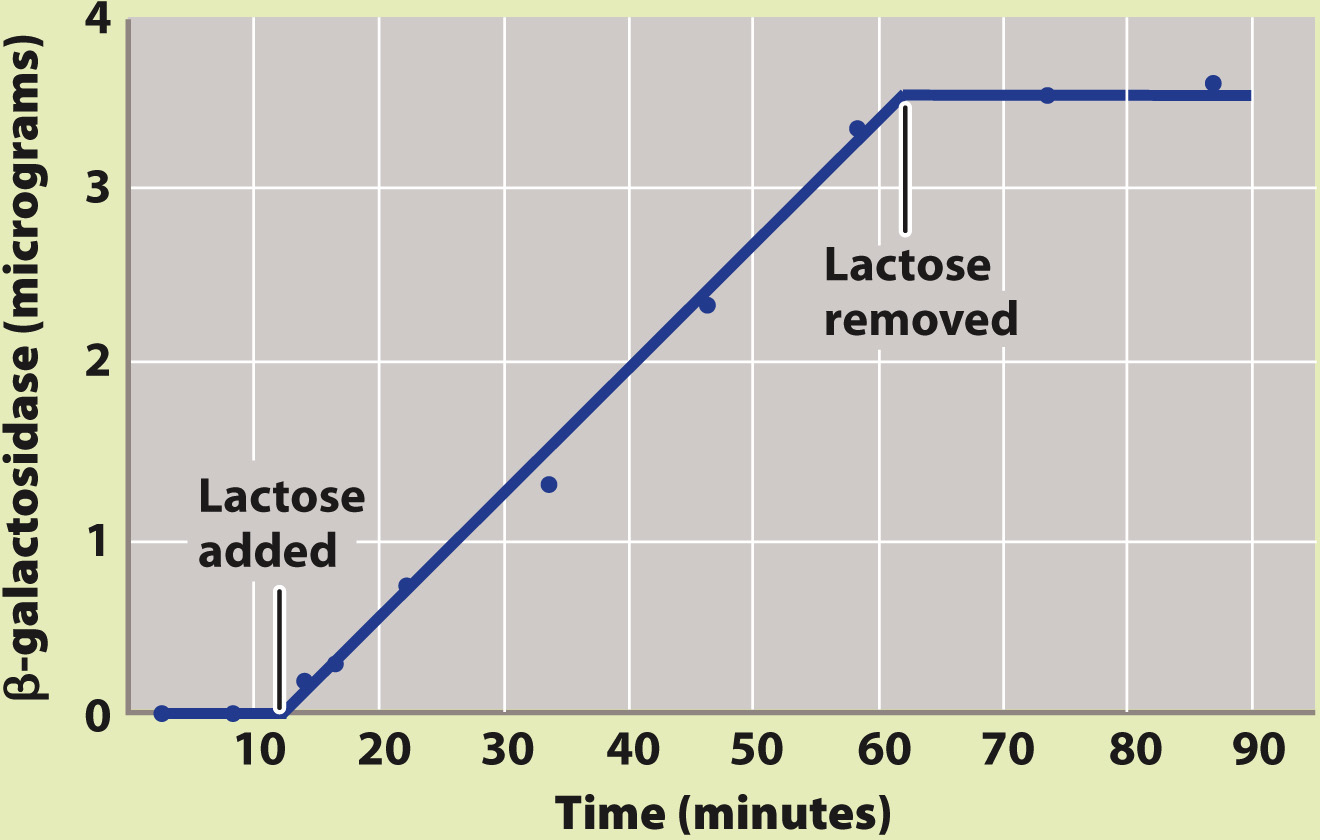
CONCLUSION Synthesis of β-galactosidase is turned on when lactose is added and turned off when lactose is removed, in support of the second hypothesis.
FOLLOW-
SOURCE Monod, J. 1965. “From Enzymatic Adaption to Allosteric Transitions.” Nobel Prize lecture. http:/
To understand how the genes for lactose utilization are regulated, you need to know the main players, and these are shown in Fig. 19.15. The overall situation looks quite complicated, but when you take it apart and look at the individual pieces, it is in fact quite simple. Let us start with the two coding sequences on the right in the figure:
- lacZ is the gene (coding sequence) for the enzyme β-galactosidase, which cleaves the lactose molecule into its glucose and galactose constituents. A functional, nonmutant form of the lacZ gene is denoted lacZ+
- lacY is the gene (coding sequence) for the protein lactose permease, which transports lactose from the external medium into the cell. A functional, nonmutant form of the lacY gene is denoted lacY+.
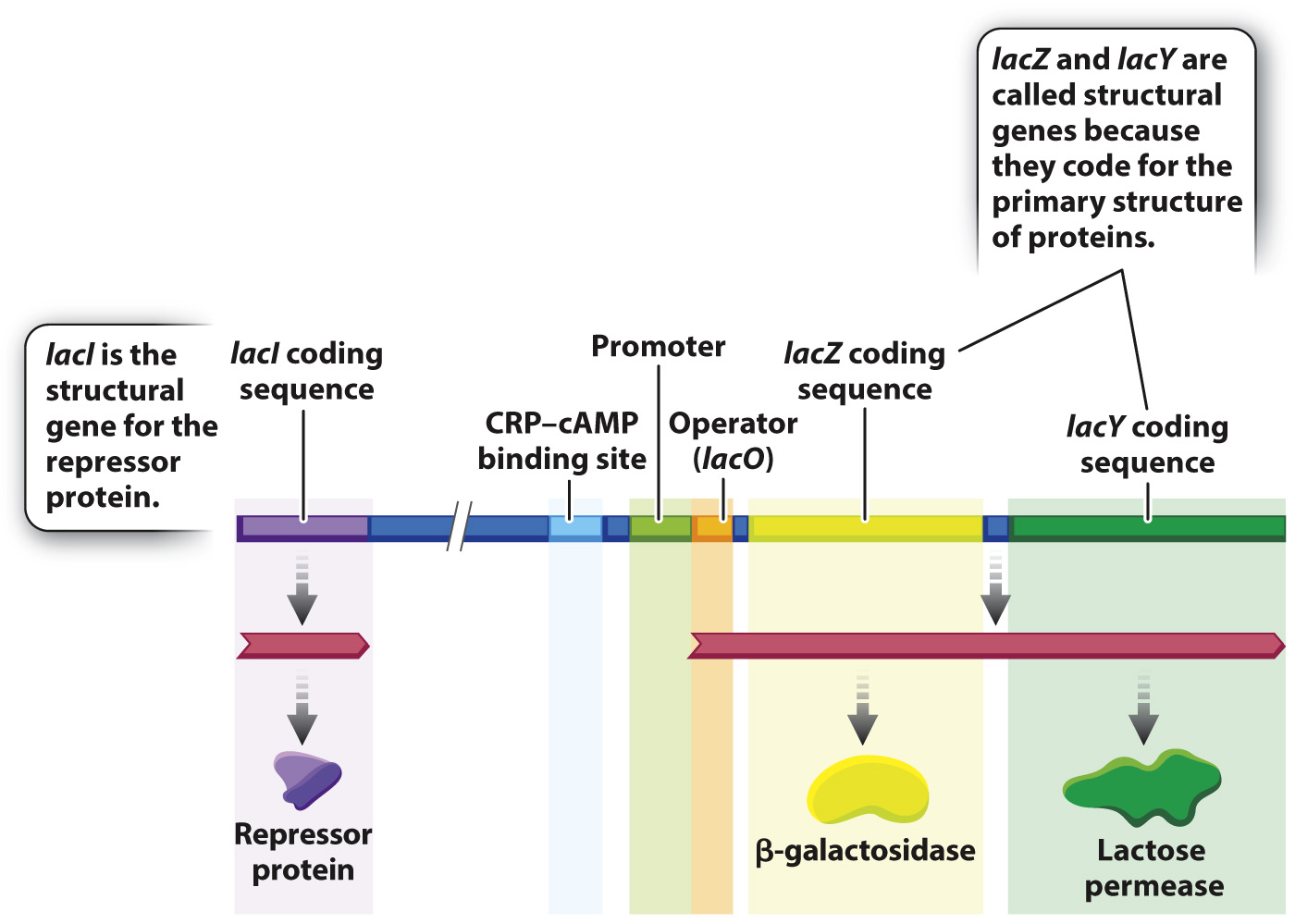
These genes are called structural genes because they code for the sequence of amino acids making up the primary structure of each protein. Bacteria that contain mutations that eliminate function of the lacZ gene (denoted lacZ– mutants), or those that eliminate function of the lacY gene (denoted lacY– mutants), cannot utilize lactose as a source of energy. Without a functional product from lacY, lactose cannot enter the cell, and without a functional product from lacZ, lactose cannot be cleaved into its component sugars. A functional form of β-galactosidase (lacZ+) and of permease (lacY+) are both essential for the utilization of lactose and for cell growth.
Regulation of the lacZ and lacY structural genes is controlled by the product of another structural gene, called lacI, which encodes a repressor protein. Located between lacI and lacZ are a series of regulatory sequences for lacZ and lacY that include a promoter, lacP, whose function is to recruit the RNA polymerase complex and initiate transcription, and an operator, lacO, which is the binding site for the repressor. Another regulatory region is a binding site for a protein called CRP, which is discussed later.
The lacZ and lacY genes share a single set of regulatory elements and are transcribed together, a gene organization that is common in bacteria. Typically, a group of functionally related genes are located next to one another along the bacterial DNA and share a promoter, and when the coding sequences of the structural genes are transcribed from the promoter, they are transcribed together into a single molecule of messenger RNA. Such an mRNA is called a polycistronic mRNA (“cistron” is an old term for “coding sequence”). In Fig. 19.15, the polycistronic RNA includes the coding sequences for β-galactosidase and lactose permease. The region of DNA consisting of the promoter, the operator, and the coding sequence for the structural genes is called an operon.
Operons are found in bacteria and archaeons, whose cells can translate polycistronic mRNA molecules correctly because their ribosomes can initiate translation anywhere along an mRNA that contains a proper ribosome-