Gene expression can be regulated at the level of an entire chromosome.
A striking example of an epigenetic form of gene regulation is the manner in which mammals equalize the expression of X-linked genes in XX females and XY males. For most genes, there is a direct relation between the number of copies of the gene (the gene dosage) and the level of expression of the gene. An increase in gene dosage increases the level of expression because each copy of the gene is regulated independently of other copies. For example, as we saw in Chapter 15, the presence of an extra copy of most human chromosomes results in spontaneous abortion because of the increase in the expression of the genes in that chromosome.
XX females have twice as many X chromosomes as XY males. For genes located in the X chromosome, the dosage of genes is twice as great in females as it is in males. However, the level of expression of X-linked genes is about the same in both sexes. These observations imply that the regulation of genes in the X chromosome is different in females and in males. This differential regulation is called dosage compensation.
Different species have evolved different mechanisms of dosage compensation. In the fruit fly Drosophila, males double the transcription of the single X chromosome to achieve equal expression compared to the two X chromosomes in females. In the nematode worm Caenorhabditis, transcription of both X chromosomes in females is decreased to one-half the level of the single X chromosome in males. In mammals, including humans, dosage compensation occurs through the inactivation of one X chromosome in each cell in females. This process, known as X-inactivation, was first proposed by Mary F. Lyon in the early 1960s.
Page 381
Soon after a fertilized egg with two X chromosomes implants in the mother’s uterine wall, one X chromosome at random is inactivated (Fig. 19.4). In Fig. 19.4a, the inactive X chromosome is shown in gray. The inactive state persists through cell division, so in each cell lineage, the same X chromosome that was originally inactivated remains inactive. The result is that a normal female is a mosaic, or patchwork, of tissue. In some patches, the genes in the maternal X chromosome are expressed (and the paternal X chromosome is inactivated), whereas in other patches, the genes in the paternal X chromosome are expressed (and the maternal X chromosome is inactivated). The term “inactive X chromosome” is a slight exaggeration since a substantial number of genes are still transcribed, although usually at a low level.
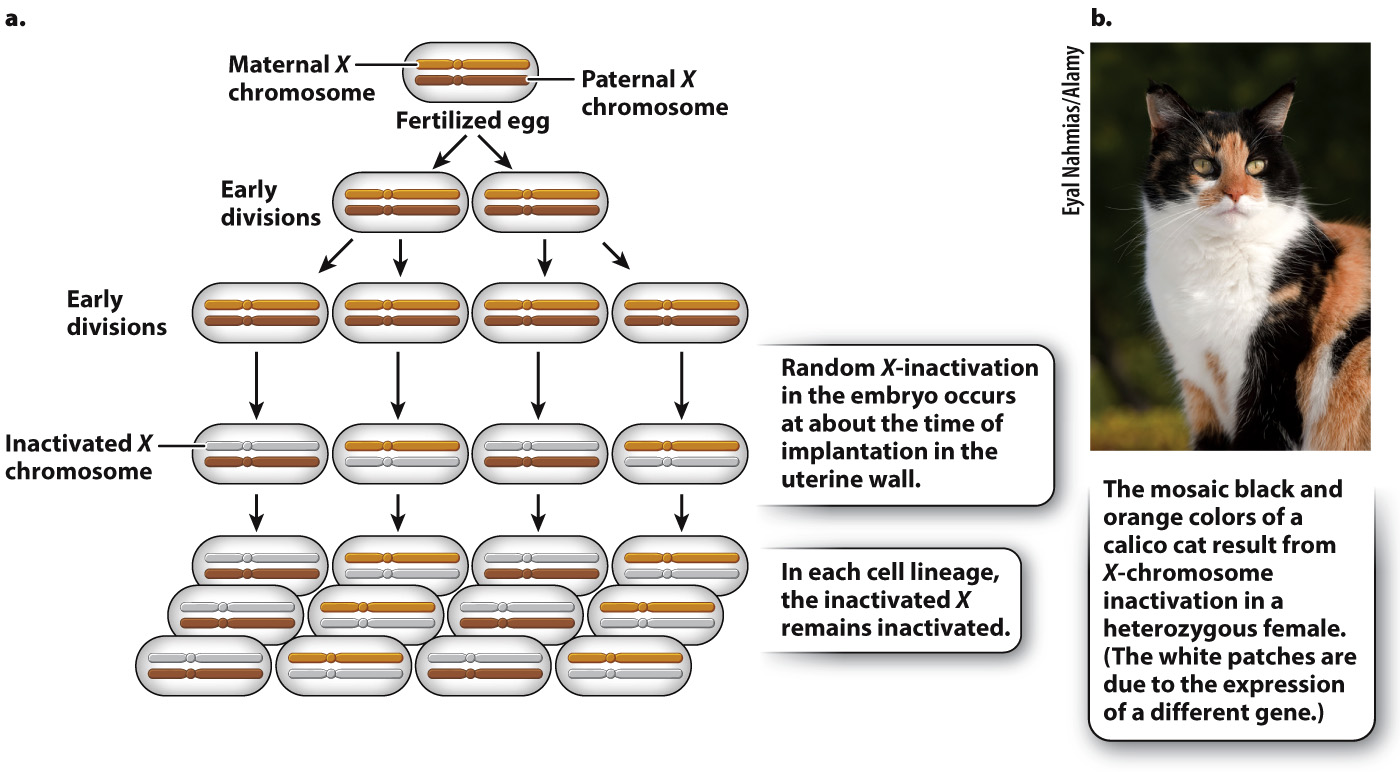
FIG. 19.4 X-inactivation in female mammals. X-inactivation equalizes the expression of most genes in the X chromosome between XX females and XY males.
As one argument for her X-inactivation hypothesis, Lyon called attention to calico cats, which are nearly always female (Fig. 19.4b). In calico cats, the orange and black fur colors are due to different alleles of a single gene in the X chromosome. In a heterozygous female, X-inactivation predicts discrete patches of orange and black, and this is exactly what is observed. (The white patches on a calico cat are due to an autosomal gene.)
How does X-inactivation work? Some of the details are still unknown, but the main features of the process are shown in Fig. 19.5. A key player is a small region in the X chromosome called the X-chromosome inactivation center (XIC), which contains a gene called Xist (X-inactivation specific transcript). The Xist gene is normally transcribed at a very low level, and the RNA is unstable, but in an X chromosome about to become inactive, Xist transcription markedly increases. The transcript undergoes RNA splicing, but it does not encode a protein. Xist RNA is therefore an example of a noncoding RNA (Chapter 3). Instead of being translated, the processed Xist RNA coats the XIC region, and as it accumulates, the coating spreads outward from the XIC until the entire chromosome is coated with Xist RNA. The presence of Xist RNA along the chromosome recruits factors that promote DNA methylation, histone modification, and other changes associated with transcriptional repression.
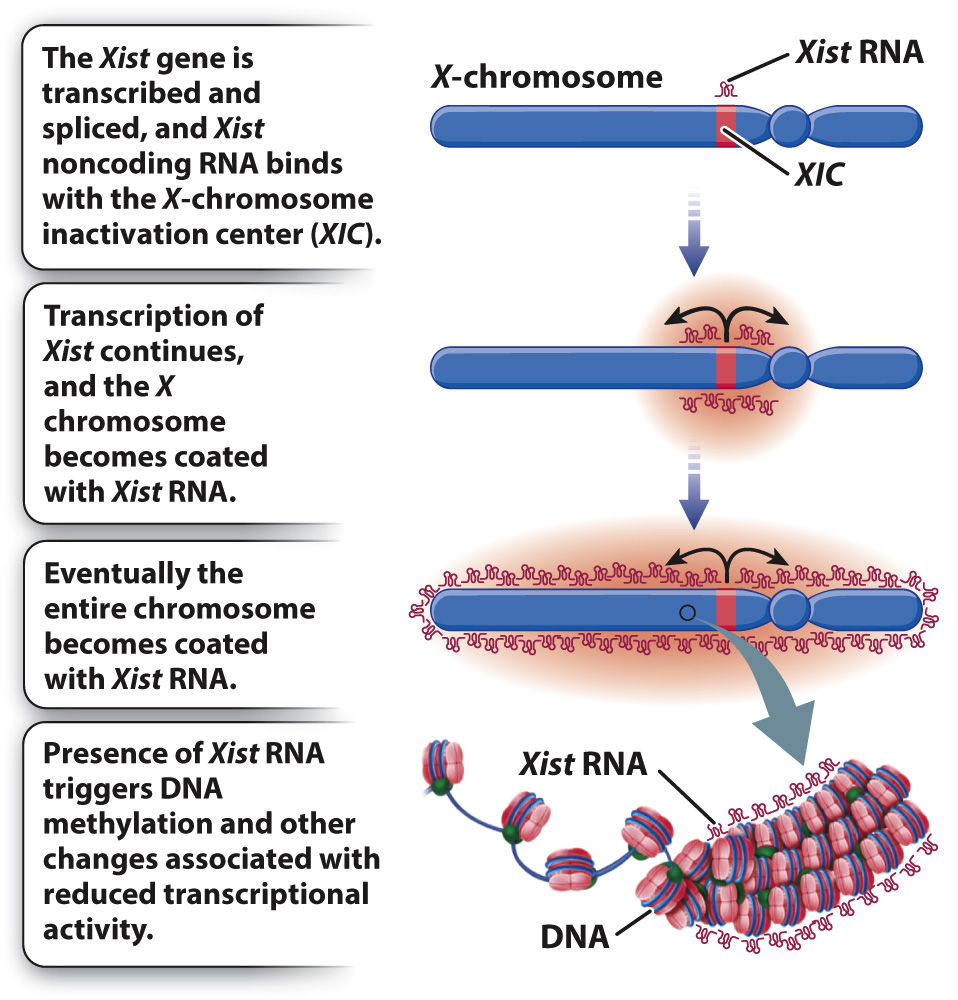
FIG. 19.5 The role of Xist in X-inactivation. The Xist transcript is a noncoding RNA that is expressed from the inactive X chromosome and coats the entire chromosome, leading to inactivation of most of the genes in the chromosome.
Page 382
The Xist gene is both necessary and sufficient for X-inactivation: If it is deleted, X-inactivation does not occur; if it is inserted into another chromosome, it inactivates that chromosome. Using recombinant DNA techniques of the sort described in Chapter 12, researchers were able to insert Xist into one of the three copies of chromosome 21 present in cells taken from a patient with Down syndrome. Remarkably, Xist in its new location in chromosome 21 produced an RNA transcript that coated that copy of chromosome 21 and repressed most transcription of its genes! Whether all genes in chromosome 21 are repressed, and whether they are as fully repressed as those in the inactive X chromosome, are questions still being investigated, but this finding raises clear (but still speculative) ideas about how Xist might be used as a potential treatment for Down syndrome and various other chromosomal disorders.

