Homeotic genes determine where different body parts develop in the organism.

Together, the segment-
Two classic examples of the consequences of mutations in homeotic genes in Drosophila are shown in Fig. 20.12. Fig. 20.12a shows what happens when the homeotic gene Antennapedia, which specifies the development of the leg, is inappropriately expressed in anterior segments. The mutation causes legs to grow where antennae usually would. Similarly, a mutation in the homeotic gene Bithorax results in the transformation of thoracic segment 3 (T3) into thoracic segment 2 (T2), so that the fruit fly has two T2 segments in a row. As shown in the Fig. 20.12b, the result is a fruit fly with two complete sets of wings.
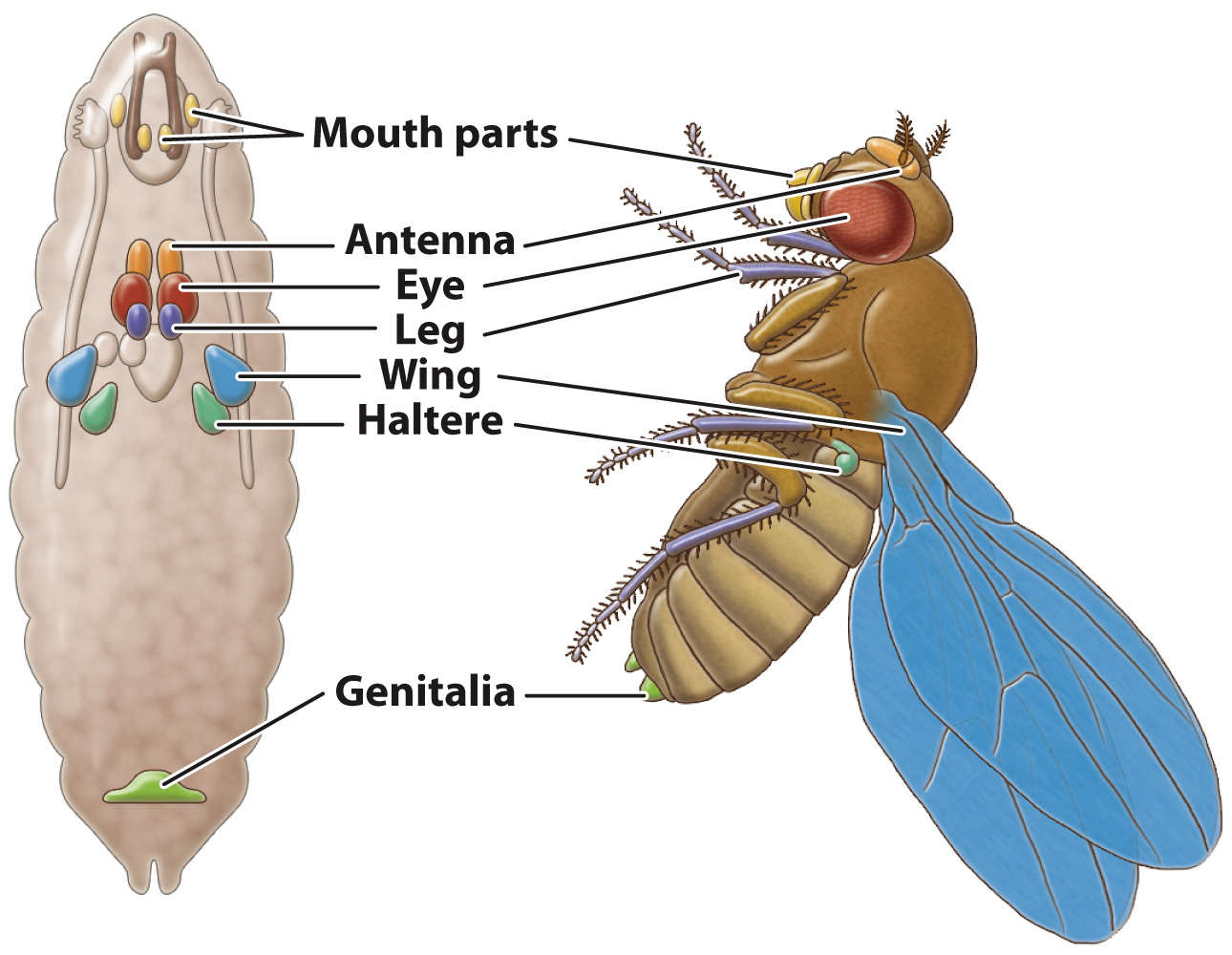
In Drosophila, the adult body parts like legs, antennae, and wings are formed from organized collections of tissue located throughout the larval body (Fig. 20.13). The development of these tissues and their metamorphosis into the adult body parts are regulated by the homeotic genes. First expressed at about the same time as the segment-
Homeotic genes encode transcription factors. The DNA-
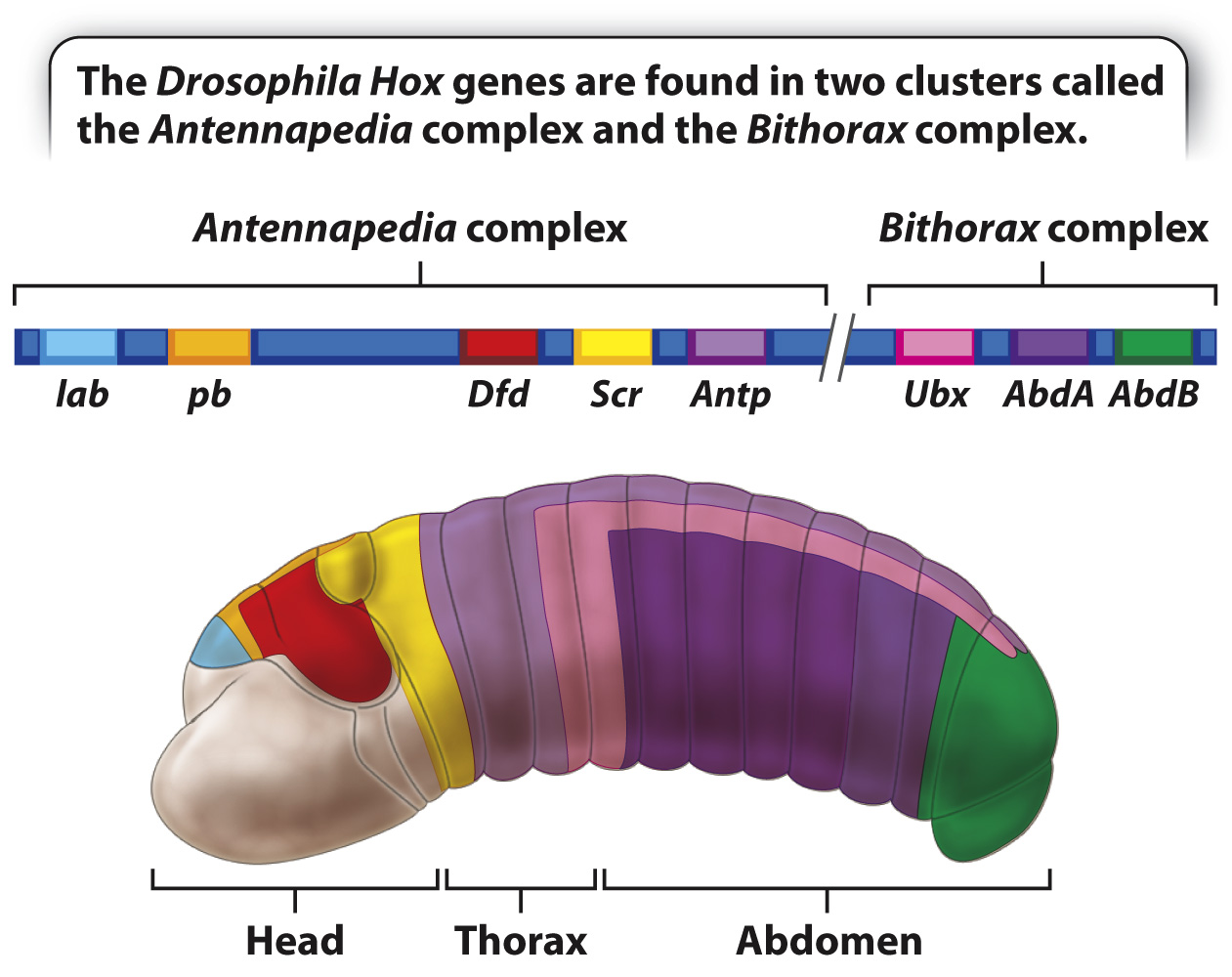
The Drosophila genome contains eight Hox genes comprising two distinct clusters, the Antennapedia complex and the Bithorax complex (Fig. 20.14). The genes are arranged along the chromosome in the same order as their products function in anterior–
Because the amino acid sequences of the homeodomains of Hox gene products are very similar from one organism to the next, Hox gene clusters have been identified in a wide variety of animals with bilateral symmetry (organisms in which both sides of the midline are mirror images), from insects to mammals. Comparison of the number and types of Hox genes in different species supports the hypothesis that the ancestral Hox gene cluster had an organization very similar to what we now see in most organisms with Hox gene clusters. In its evolutionary history, the vertebrate genome underwent two whole-
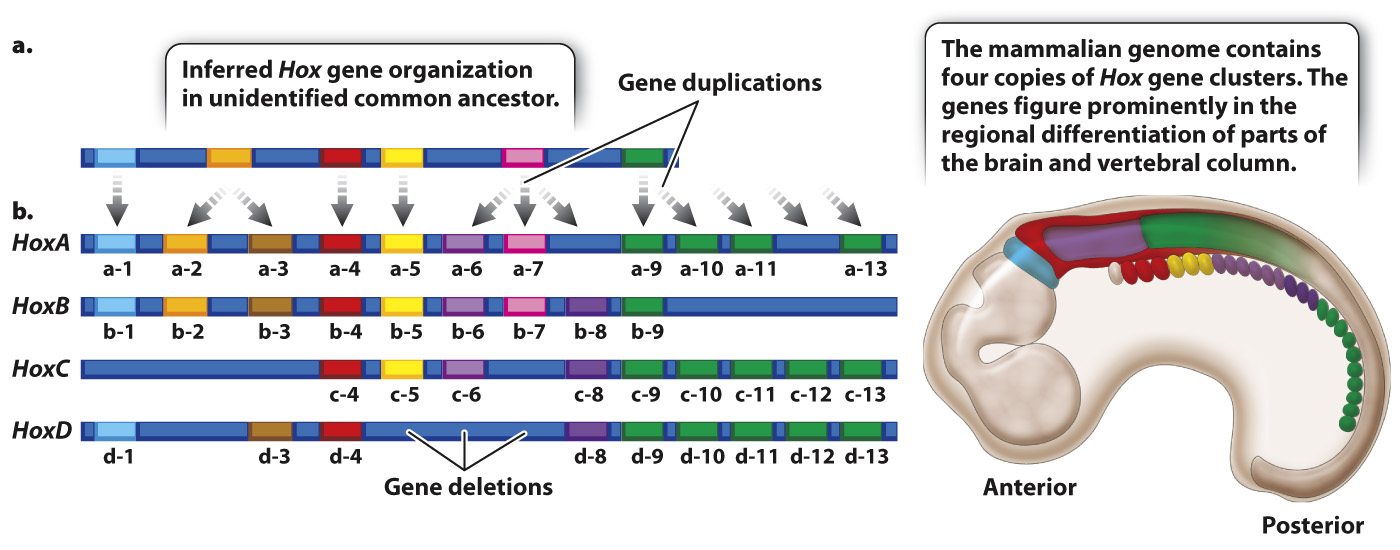
Unlike the Hox genes in Drosophila, mammalian Hox genes do not specify limbs but are important in the embryonic development of structures that become parts of the hindbrain, spinal cord, and vertebral column (Fig. 20.15). As in Drosophila, the genes in each cluster are expressed according to their linear order along the chromosome, which coincides with the linear order of regions the genes affect in the embryo. Each gene helps to specify the identity of the region in which it is expressed. Many of the genes in the mammalian Hox clusters have redundant or overlapping functions so that learning exactly what each gene does continues to be a research challenge. The evolutionary and developmental study of Hox gene conservation and expression is a good example of recent evo-