Geological data indicate the age and environmental setting of fossils.
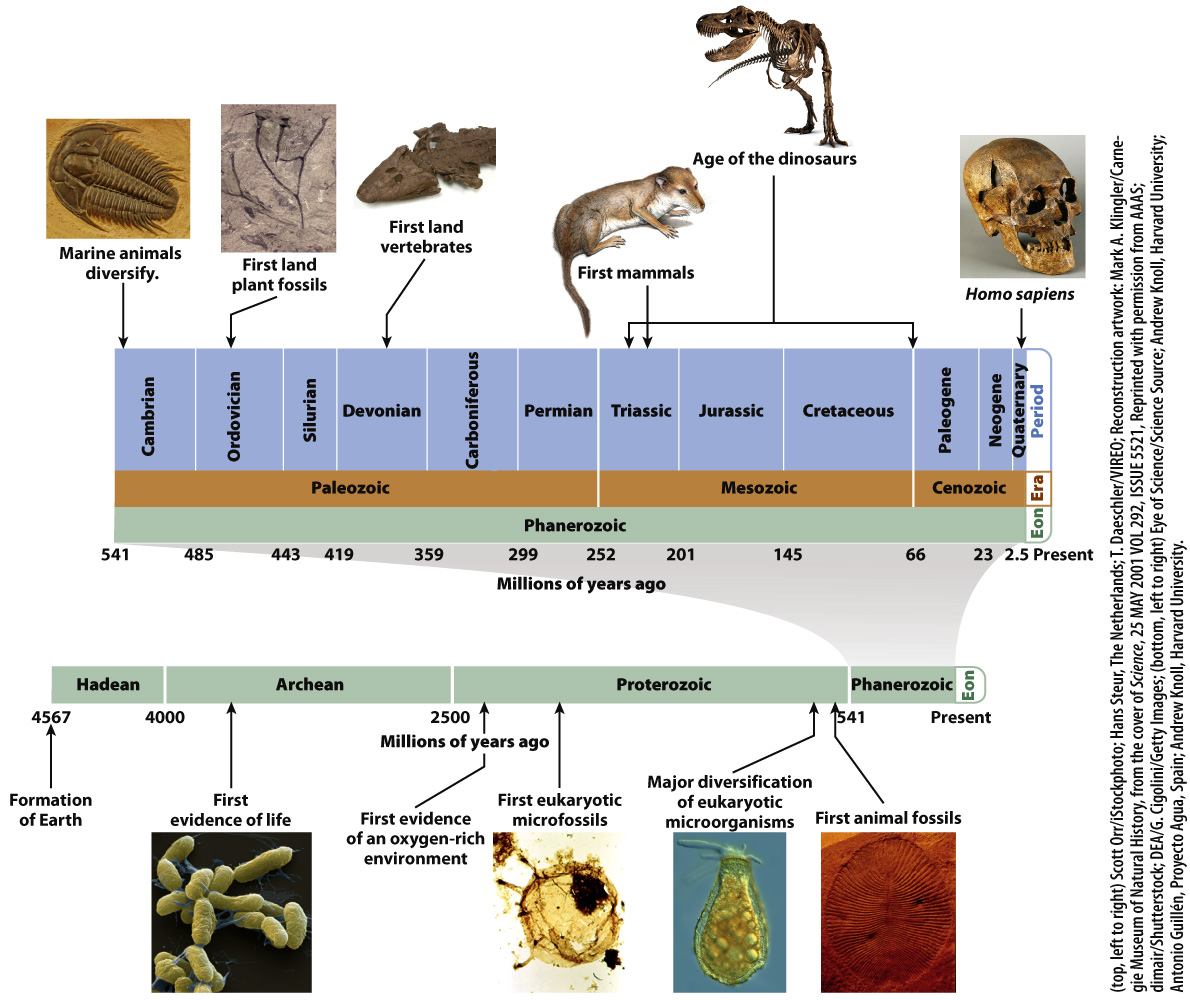
How do we know the age of a fossil? Beginning in the nineteenth century, geologists recognized that groups of fossils change systematically from the bottom of a sedimentary rock formation to its top. As more of Earth’s surface was mapped and studied, it became clear that certain fossils always occur in layers that lie beneath (and so are older than) layers that contain other species. From these patterns, geologists concluded that fossils mark time in Earth history. At first, geologists didn’t know why fossils changed from one bed to the next, but after Darwin the reason became apparent: Fossils record the evolution of life on Earth. They eventually mapped out the geologic timescale, the series of time divisions that mark Earth’s long history (Fig. 23.16).
The layers of fossils in sedimentary rocks can tell us that some rocks are older than others, but they cannot by themselves provide an absolute age. Calibration of the timescale became possible with the discovery of radioactive decay. In Chapter 2, we discussed isotopes, variants of an element that differ from one another in the number of neutrons they contain. Many isotopes are unstable and spontaneously break down to form other, more stable isotopes. In the laboratory, scientists can measure how fast unstable isotopes decay. Then, by measuring the amounts of the unstable isotope and its stable daughter inside a mineral, they can determine when the mineral formed.
Archaeologists commonly use the radioactive decay of the isotope carbon-
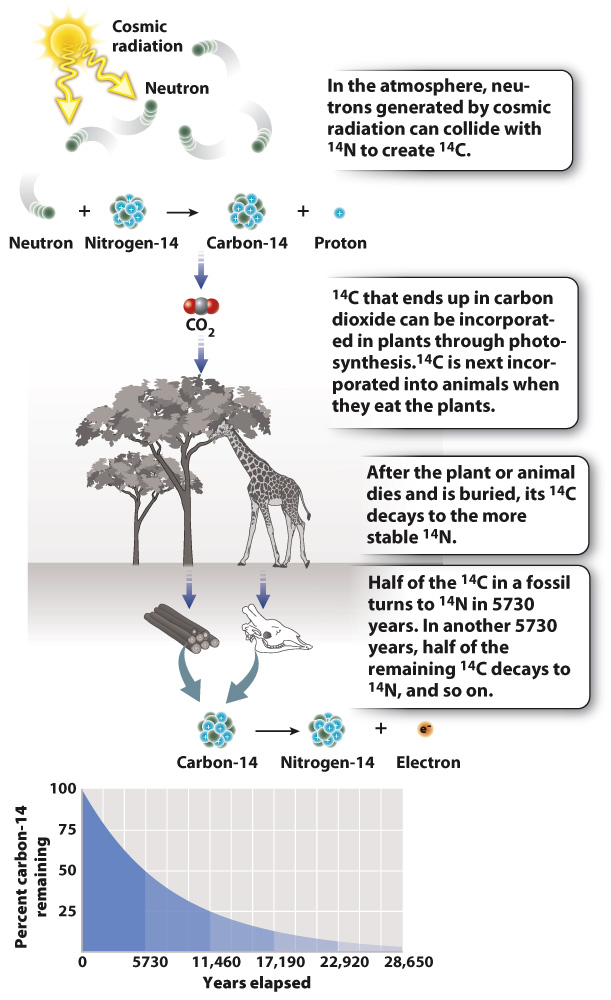
Because its half-
The sedimentary rocks that contain fossils also preserve, encrypted in their physical features and chemical composition, information about the environment in which they formed. Sandstone beds, for example, may have rippled surfaces, like the ripples produced by currents that we see today in the sand of a seashore or lake margin. Pyrite (FeS2), or fool’s gold, forms when H2S generated by anaerobic bacteria reacts with iron. As these conditions generally occur where oxygen is absent, pyrite enrichment in ancient sedimentary rocks can signal oxygen depletion.
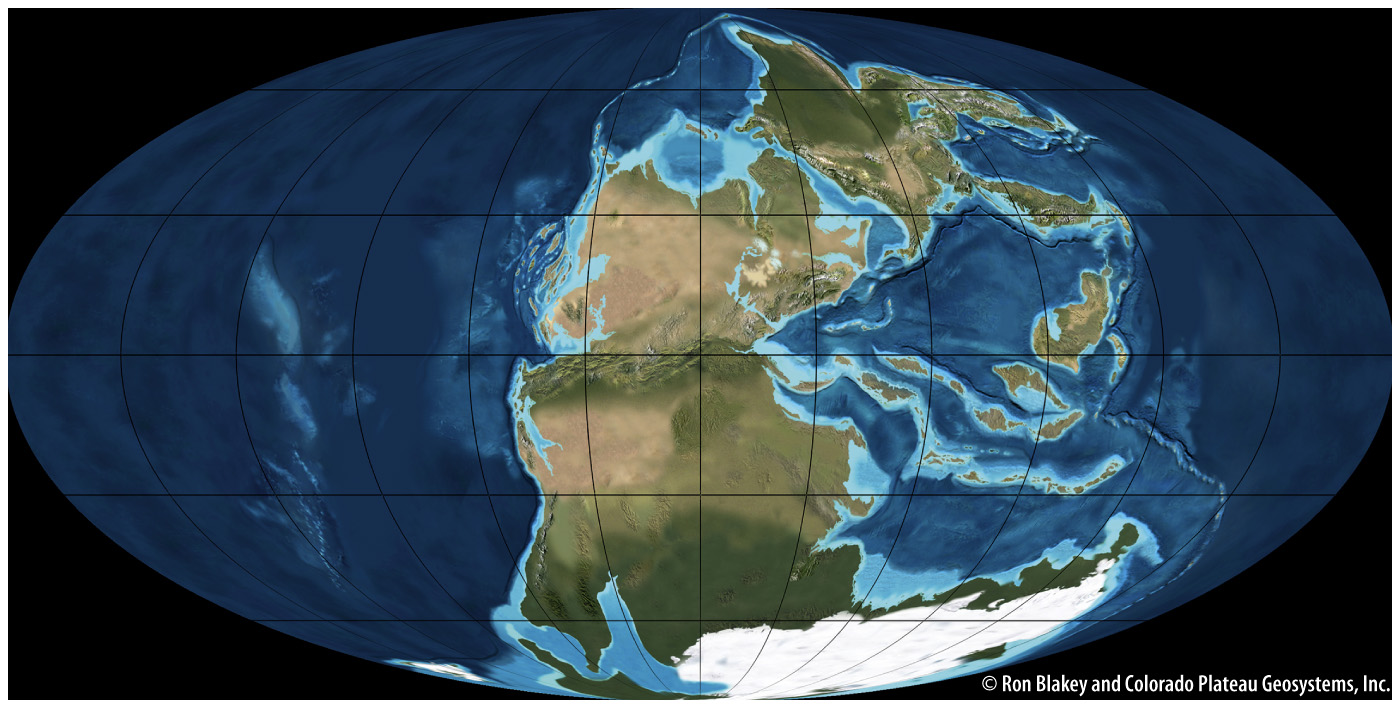
We might think our moment in geologic time is representative of Earth as it has always existed, but nothing could be further from the truth. In the location and sizes of its continents, ocean chemistry, and atmospheric composition, the Earth we experience is unlike any previous state of the planet. Today, for example, the continents are distributed widely over the planet’s surface, but 290 million years ago they were clustered in a supercontinent called Pangaea (Fig. 23.18). Oxygen gas permeates most surface environments of Earth today, but 3 billion years ago, there was no O2 anywhere. And, just 20,000 years ago, 2 km of glacial ice stood where Boston lies today. Sedimentary rocks record the changing state of Earth’s surface over billions of years and show that life and environment have changed together through time, each influencing the other.