Bacteria and archaeons dominate Earth’s sulfur cycle.
Our bodies are mostly carbon, oxygen, and hydrogen, but we also contain about 0.2% sulfur by weight. Sulfur is a component of the amino acids cysteine and methionine and therefore is present in many proteins (Chapter 2). Sulfur is also present in iron–
Plants are the dominant primary producers on land. Plants take up sulfate (SO42–) ions from the soil and reduce them within their cells to hydrogen sulfide (H2S) that can be incorporated into cysteine and other biomolecules (Fig. 26.10). This process is called assimilation. Algae and photosynthetic bacteria do much the same thing in lakes, rivers, and oceans. Why don’t primary producers take up H2S directly? There are two answers to this question. First, H2S is rapidly oxidized in the presence of oxygen and so does not occur in environments where oxygenic photosynthesis is common. Second, H2S is generally toxic to eukaryotic organisms, so plants and algae do not thrive where it is abundant. (The H2S produced within eukaryotic cells has a short lifetime and is restricted to intracellular sites distant from those that are vulnerable to its toxic effects.)
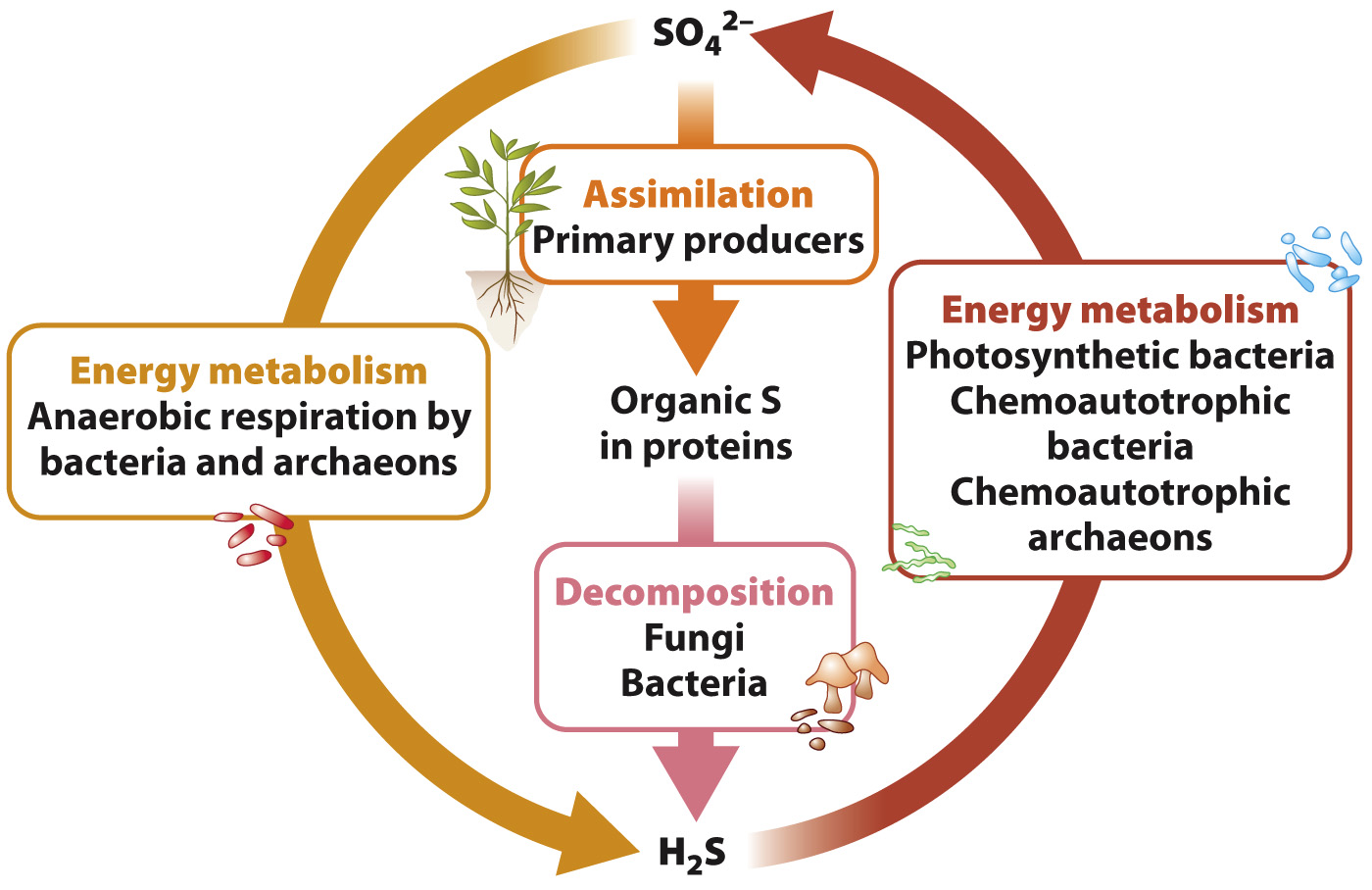
We’ve now seen half the sulfur cycle, the conversion of sulfate to H2S within cells. How did sulfate molecules get into the soil in the first place? After death, fungi and bacteria decompose cells, returning carbon, sulfur, and other compounds to the environment. Reduced sulfur compounds released from decomposing cells are oxidized by bacteria and archaeons, completing the cycle. These microbes are chemoautotrophs that obtain energy by oxidizing H2S or photosynthesizers that use H2S as the electron donor. In addition to being taken up by plants, the sulfate produced by these processes is consumed by heterotrophic bacteria living in oxygen-
Note that eukaryotes use neither H2S for photo-