The nitrogen cycle is also driven by bacteria and archaeons.
The model provided by the sulfur cycle can be extended to other biologically important elements. Nitrogen is the fourth most abundant element in the human body, contributing 4% by weight as a component of proteins, nucleic acids, and other compounds. As is true of sulfur, primary producers incorporate inorganic forms of nitrogen into biomolecules, and we get the nitrogen we need from our food. As we’ll see, however, there is a twist.
Let’s examine the biological nitrogen cycle (Fig. 26.11). Carbon is a minor component of the atmosphere and sulfur only a trace constituent, but the air we breathe consists mostly (78%) of nitrogen gas (N2). Indeed, most of the nitrogen at Earth’s surface resides in the atmosphere. Although nitrogen is plentiful, the nitrogen available to primary producers in many environments is limited. The reason is that plants cannot make use of nitrogen gas and neither can algae. Only certain bacteria and archaeons can reduce N2 to ammonia (NH3), a form of nitrogen that can be incorporated into biomolecules.
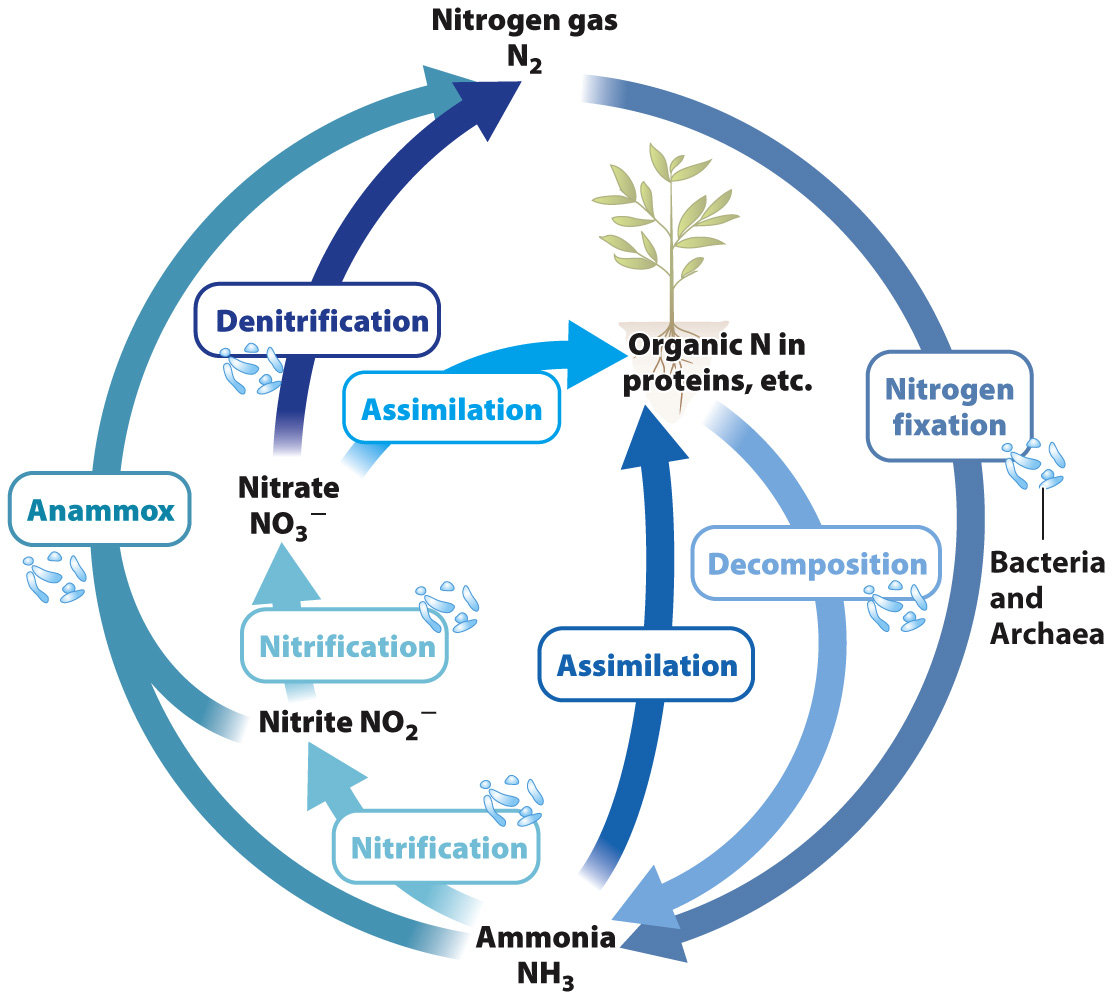
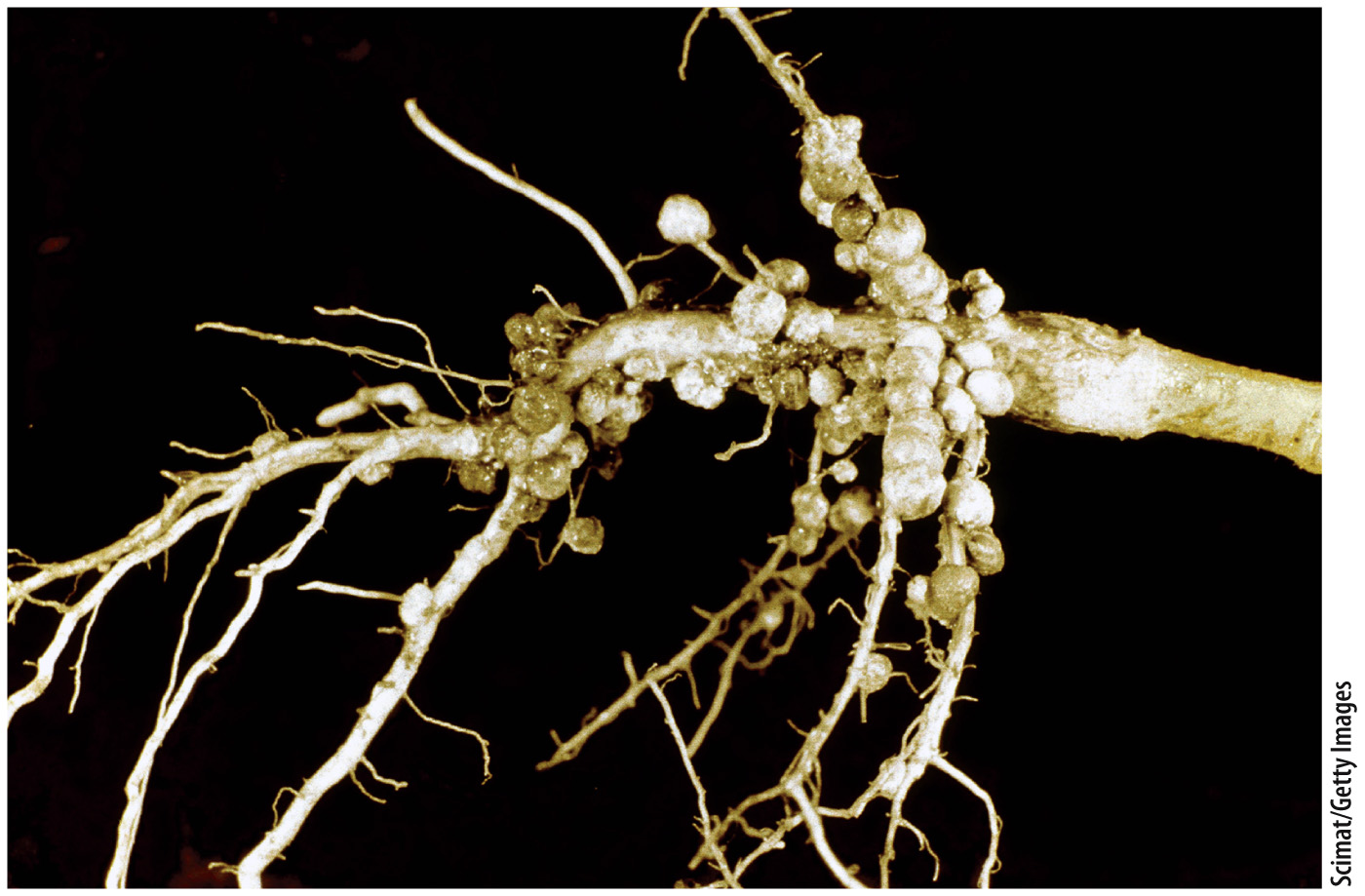
The process of converting N2 into a biologically useful form such as ammonia is called nitrogen fixation, and it is one of the most important reactions ever evolved by organisms. Farmers plant soybeans to regenerate nitrogen nutrients in soils, but it is not the soybean that fixes nitrogen. Rather, soybean roots have nodules that harbor nitrogen-
The nitrogen cycle, then, begins with nitrogen fixation, and it continues through many additional metabolisms, most of them confined to bacteria and archaeons. Ammonia released by the decomposition of dead cells can be taken up by primary producers, but commonly it is oxidized to nitrate (NO3–) by chemoautotrophic bacteria, a process called nitrification (see Fig. 26.11). This process works efficiently in both soil and water, and so nitrate is the most common form of nitrogen available to life in both environments.
Anaerobic respiration completes the cycle by returning N2 to the atmosphere. Some bacteria can use nitrate as an electron acceptor in respiration, a process known as denitrification (see Fig. 26.11). Because denitrification is so efficient at returning nitrogen to the atmosphere as nitrogen gas, nitrogen fixation must continually make nitrogen available for organisms. Without nitrogen fixation, the nitrogen cycle would slowly wind down, and the productivity of Earth’s biota—
Until the 1990s, the nitrogen cycle was described—
NH4+ + NO2– → N2 + 2H2O
Note that this is a redox reaction, like other reactions involved in energy metabolism. In this case, the ammonium ion is oxidized to nitrogen gas, and nitrite is reduced to nitrogen gas, with water produced as a by-
The biological nitrogen cycle, then, begins and ends with prokaryotic metabolisms. Bacteria and archaeons fix N2 into a biologically available form, nitrifying bacteria oxidize ammonia to nitrate, and still other prokaryotes gain energy from denitrification and anammox, returning N2 to the air. Unlike the carbon and sulfur cycles, the nitrogen cycle does not rely at any point on elements stored as minerals in rocks and sediments. Most of Earth’s nitrogen was released to the atmosphere as gas early in our planet’s history, and so movement into sediments and out of volcanoes contributes relatively little to the cycle. Humans, however, contribute in important ways, most notably through the industrial synthesis of nitrate fertilizer (Chapter 49).
Quick Check 5 How would the nitrogen cycle operate in the absence of bacteria and archaeons?
Quick Check 5 Answer
It wouldn’t. Bacteria and archaeons cycle nitrogen between the atmosphere (nitrogen gas) and biologically useful forms. In the absence of these prokaryotes, only a limited amount of nitrogen would be fixed into biologically useful form by lightning, greatly limiting the extent of life.