A hydrogen bond is an interaction between a hydrogen atom and an electronegative atom.
Because the oxygen and hydrogen atoms have slight charges, water molecules orient themselves to minimize the repulsion of like charges so that positive charges are near negative charges. For example, because of its slight positive charge, a hydrogen atom in a water molecule tends to orient toward the slightly negatively charged oxygen atom in another molecule.
A hydrogen bond is the name given to this interaction between a hydrogen atom with a slight positive charge and an electronegative atom of another molecule. In fact, any hydrogen atom covalently bound to an electronegative atom (such as oxygen or nitrogen) will have a slight positive charge and can form a hydrogen bond. In the case of water, the result of many such interactions is a molecular network stabilized by hydrogen bonds. Typically, a hydrogen bond is depicted by a dotted line, as in Fig. 2.10.
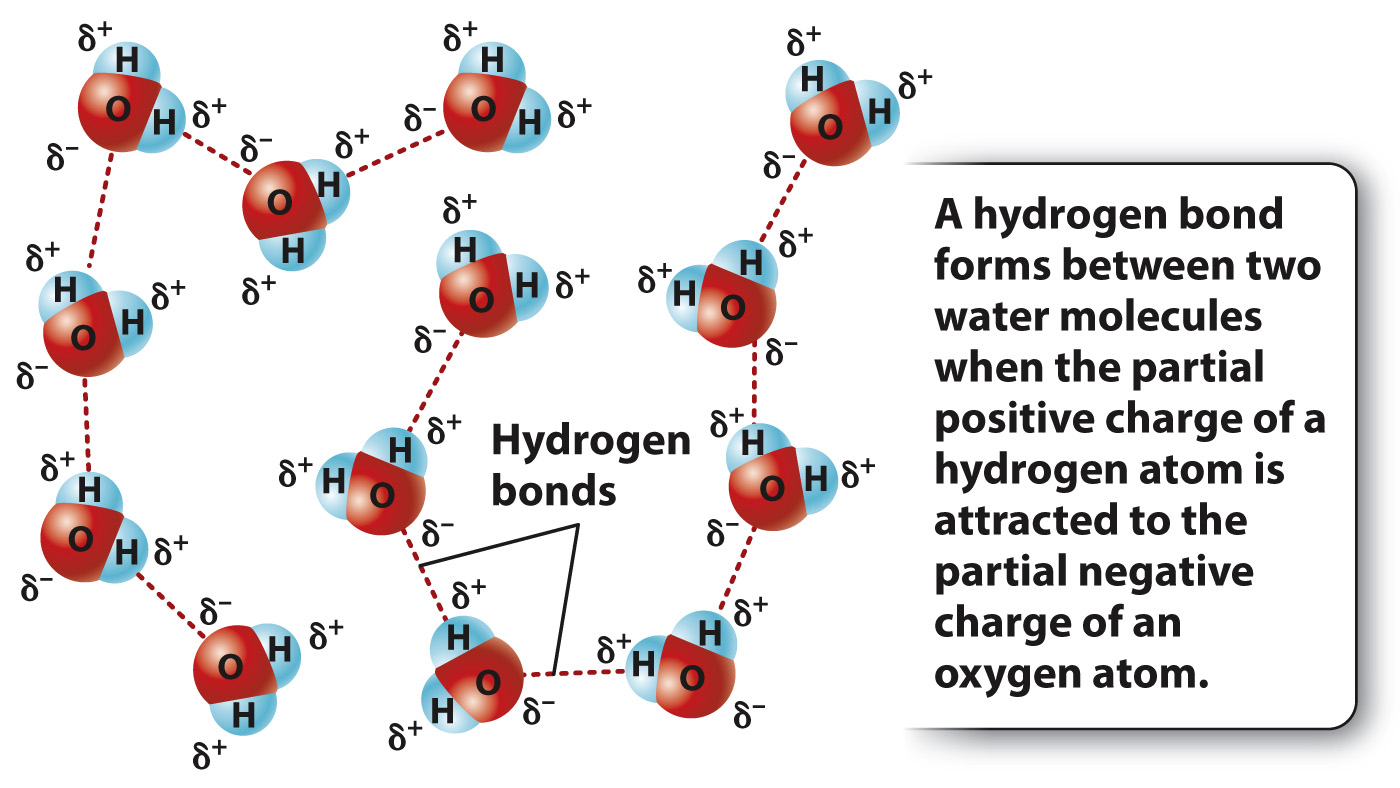
Hydrogen bonds are much weaker than covalent bonds, but it is hydrogen bonding that gives water many interesting properties, described next. In addition, the presence of many weak hydrogen bonds can help stabilize biological molecules, as in the case of nucleic acids and proteins.