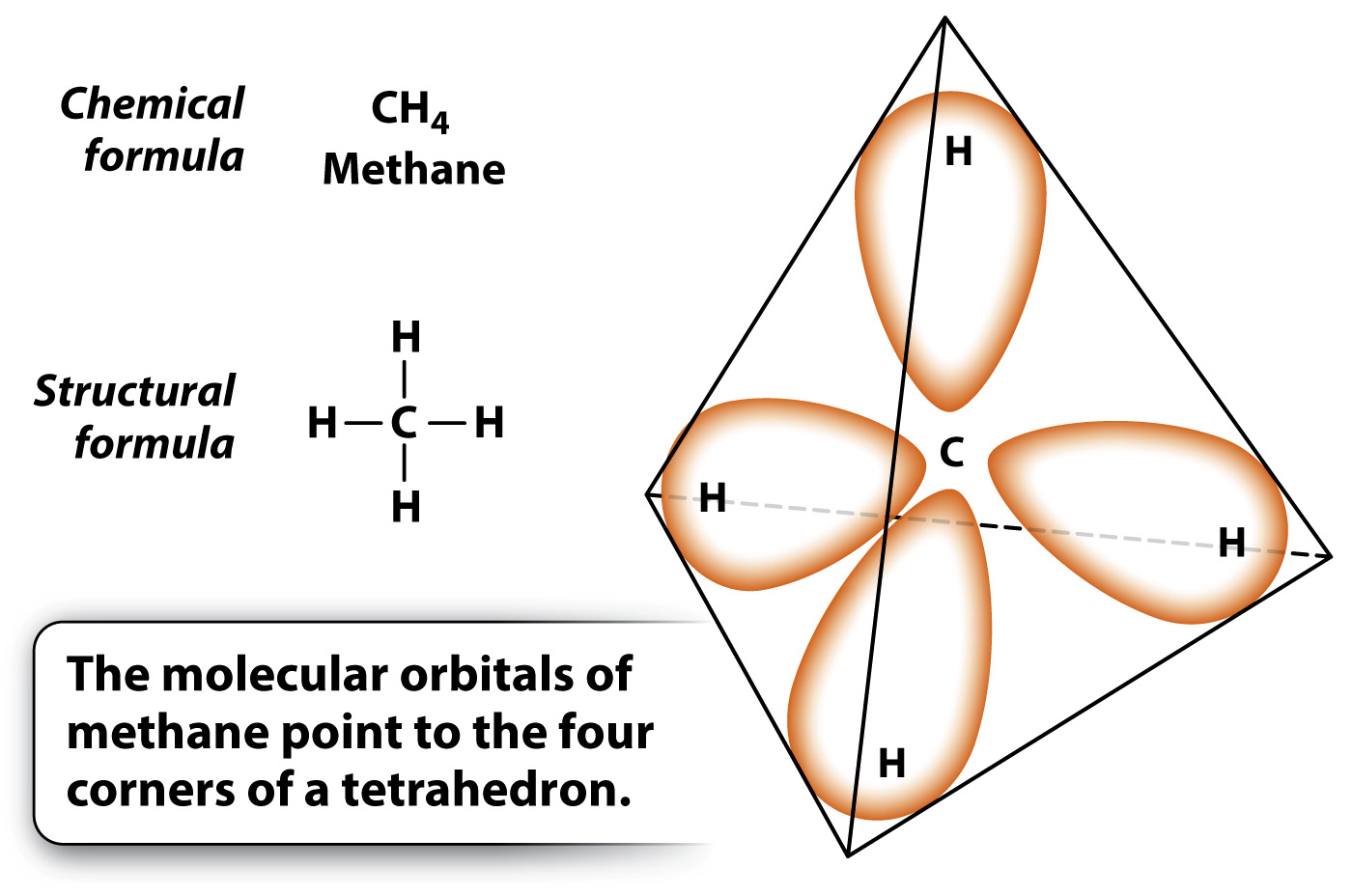
Carbon atoms form four covalent bonds.
One of the special properties of carbon is that, in forming molecular orbitals, a carbon atom behaves as if it had four unpaired electrons. This behavior occurs because one of the electrons in the outermost spherical orbital moves into the empty dumbbell-
Fig. 2.13 shows the molecular orbitals that result when one atom of carbon combines with four atoms of hydrogen to form the gas methane (CH4). Each of the four valence electrons of carbon shares a new molecular orbital with the electron of one of the hydrogen atoms. These bonds can rotate freely about their axis. Furthermore, because of the shape of the orbitals, the carbon atom lies at the center of a three-