Complex carbohydrates are made up of simple sugars.
Many of us, when we feel tired, reach for a candy bar for a quick energy boost. The energy in a candy bar comes from sugars, which are quickly broken down to release energy. Sugars belong to a class of molecules called carbohydrates, distinctive molecules composed of C, H, and O atoms, usually in the ratio 1:2:1. Carbohydrates provide a principal source of energy for metabolism.
The simplest carbohydrates are sugars (also called saccharides). Simple sugars are linear or, far more commonly, cyclic molecules containing five or six carbon atoms. All 6-
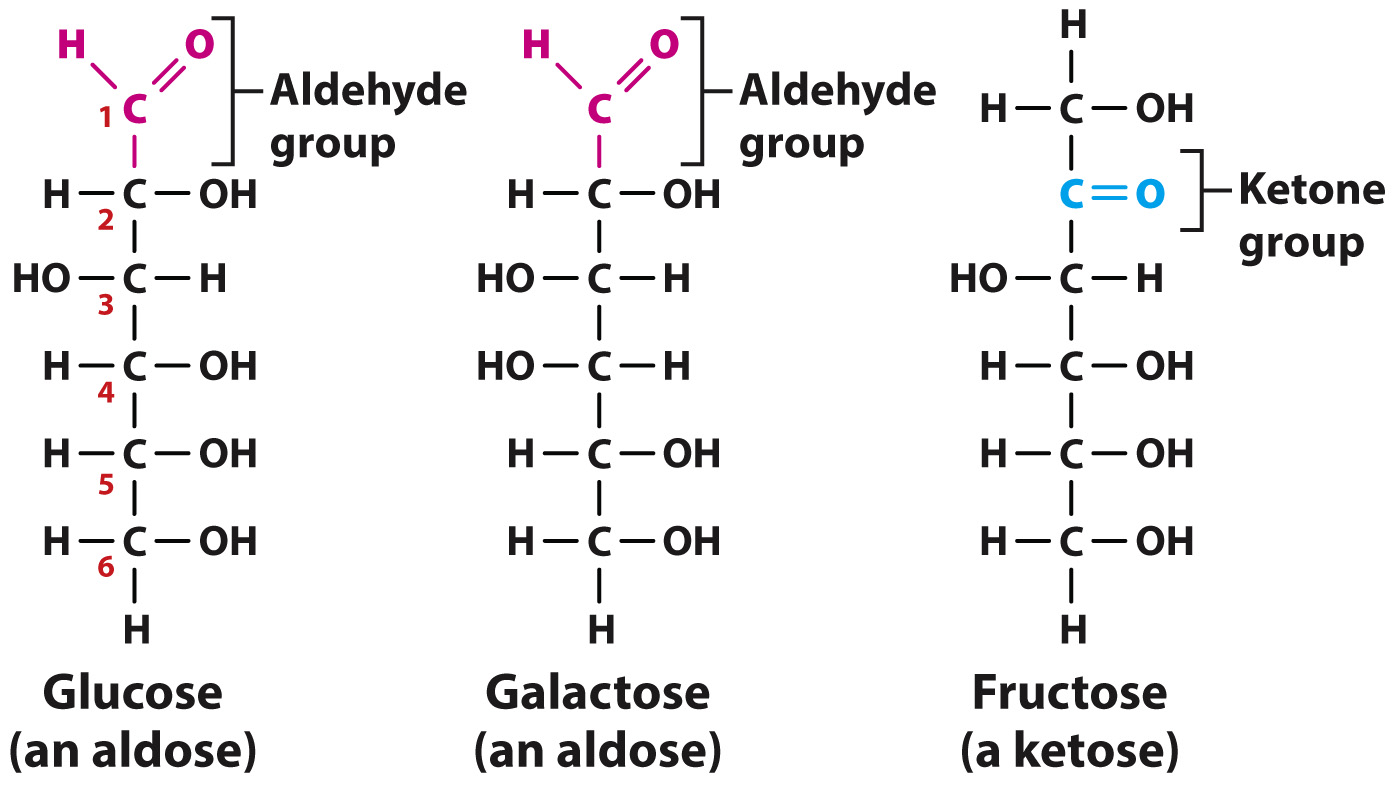
Quick Check 4 Take a close look at Fig. 2.22. How is glucose different from galactose?
Quick Check 4 Answer
Glucose and galactose differ only in the orientation of the –OH and –H groups attached to carbon 4.
A simple sugar is also called a monosaccharide (mono means “one”), and two simple sugars linked together by a covalent bond is called a disaccharide (di means “two”). Sucrose (C12H22O11), or table sugar, is a disaccharide that combines one molecule each of glucose and fructose. Simple sugars combine in many ways to form polymers called polysaccharides (poly means “many”) that provide long-
Let’s take a closer look at monosaccharides, the simplest sugars. Monosaccharides are unbranched carbon chains with either an aldehyde (HC = O) or a ketone (C = O) group (Fig. 2.22). Monosaccharides with an aldehyde group are called aldoses and those with a ketone group are known as ketoses. In both types of monosaccharide, the other carbons each carry one hydroxyl (–OH) group and one hydrogen (H) atom. When the linear structure of a monosaccharide is written with the aldehyde or ketone group at the top, the carbons are numbered from top to bottom.
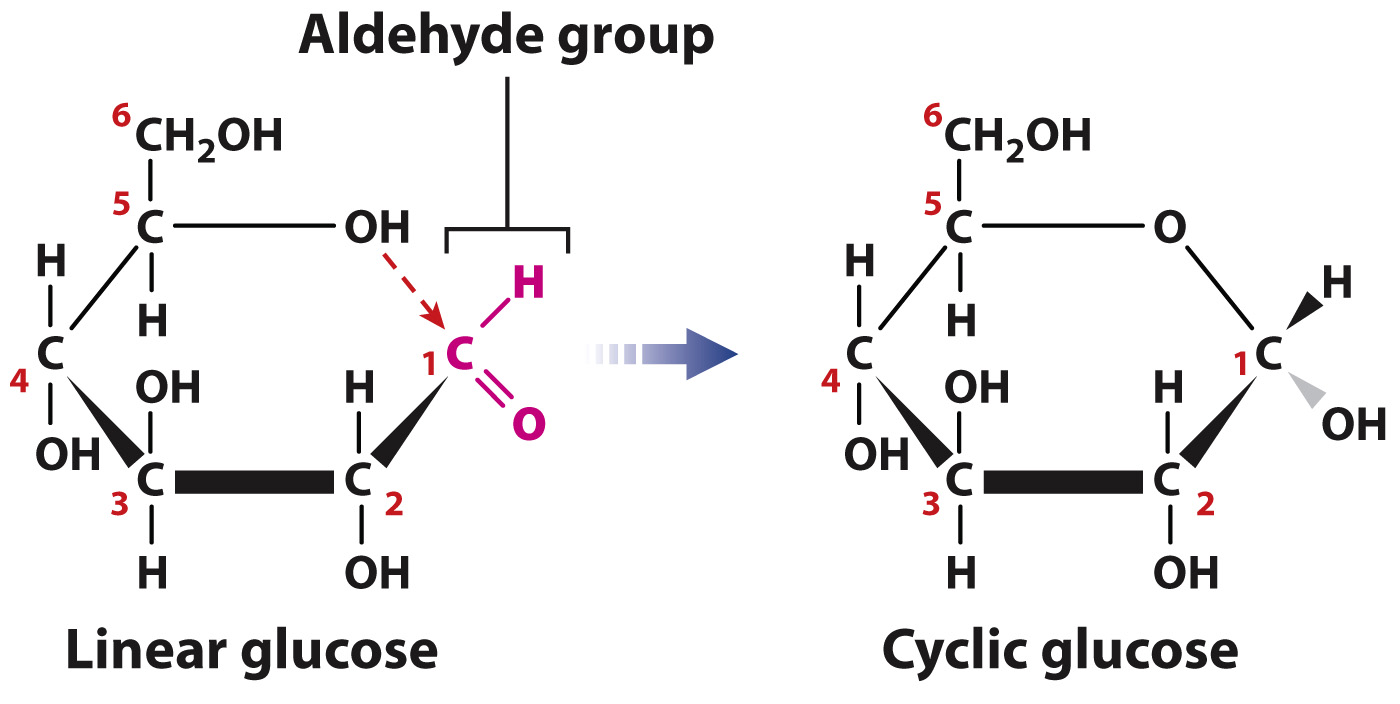
Virtually all of the monosaccharides in cells are in ring form (Fig. 2.23), not linear structures. To form a ring, the carbon in the aldehyde or ketone group forms a covalent bond with the oxygen of a hydroxyl group carried by another carbon in the same molecule. For example, cyclic glucose is formed when the oxygen atom of the hydroxyl group on carbon 5 forms a covalent bond with carbon 1, which is part of an aldehyde group. The cyclic structure is approximately flat, and you can visualize it perpendicular to the plane of the paper with the covalent bonds indicated by the thick lines in the foreground. The groups attached to any carbon therefore project either above or below the ring. When the ring is formed, the aldehyde oxygen becomes a hydroxyl group. The presence of the polar hydroxyl groups through the sugar ring makes these molecules highly soluble in water.
Monosaccharides, especially 6-
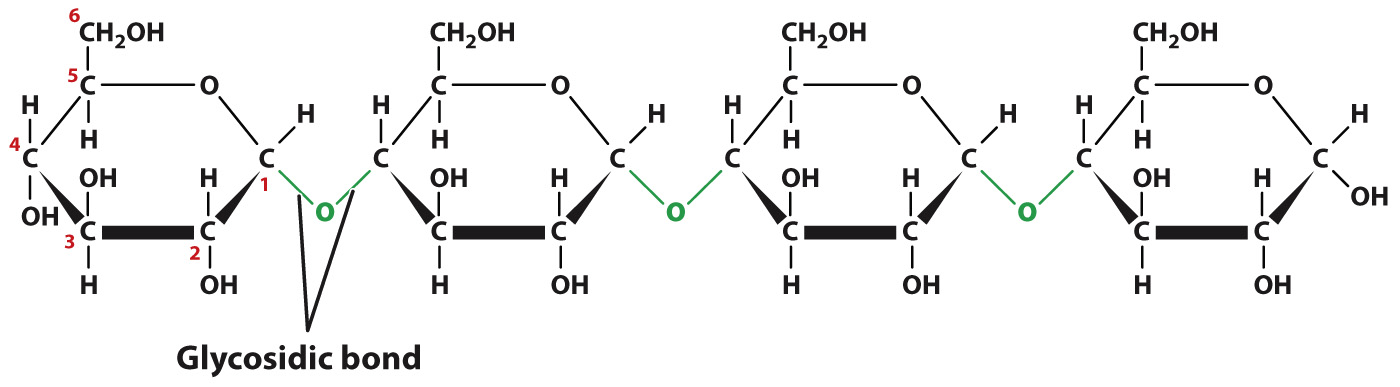
Carbohydrate diversity stems in part from the monosaccharides that make up carbohydrates, similar to the way that protein and nucleic acid diversity stems from the sequence of their subunits. Some complex carbohydrates are composed of a single type of monosaccharide, while others are a mix of different kinds of monosaccharide. Starch, for example, is a sugar storage molecule in plants composed completely of glucose molecules, whereas pectin, a component of the cell wall, contains up to five different monosaccharides.