Lipids are hydrophobic molecules.
Proteins, nucleic acids, and carbohydrates all are polymers made up of smaller, repeating units with a defined structure. Lipids are different. Instead of being defined by a chemical structure, they share a particular property: Lipids are all hydrophobic. Because they share a property and not a structure, lipids are a chemically diverse group of molecules. They include familiar fats that make up part of our diet, components of cell membranes, and signaling molecules. Let’s briefly consider each in turn.
Triacylglycerol is an example of a lipid that is used for energy storage. It is the major component of animal fat and vegetable oil. A triacylglycerol molecule is made up of three fatty acids joined to glycerol (Fig. 2.25). A fatty acid is a long chain of carbon atoms attached to a carboxyl group (–COOH) at one end (Figs. 2.25a and 2.25b). Glycerol is a 3-
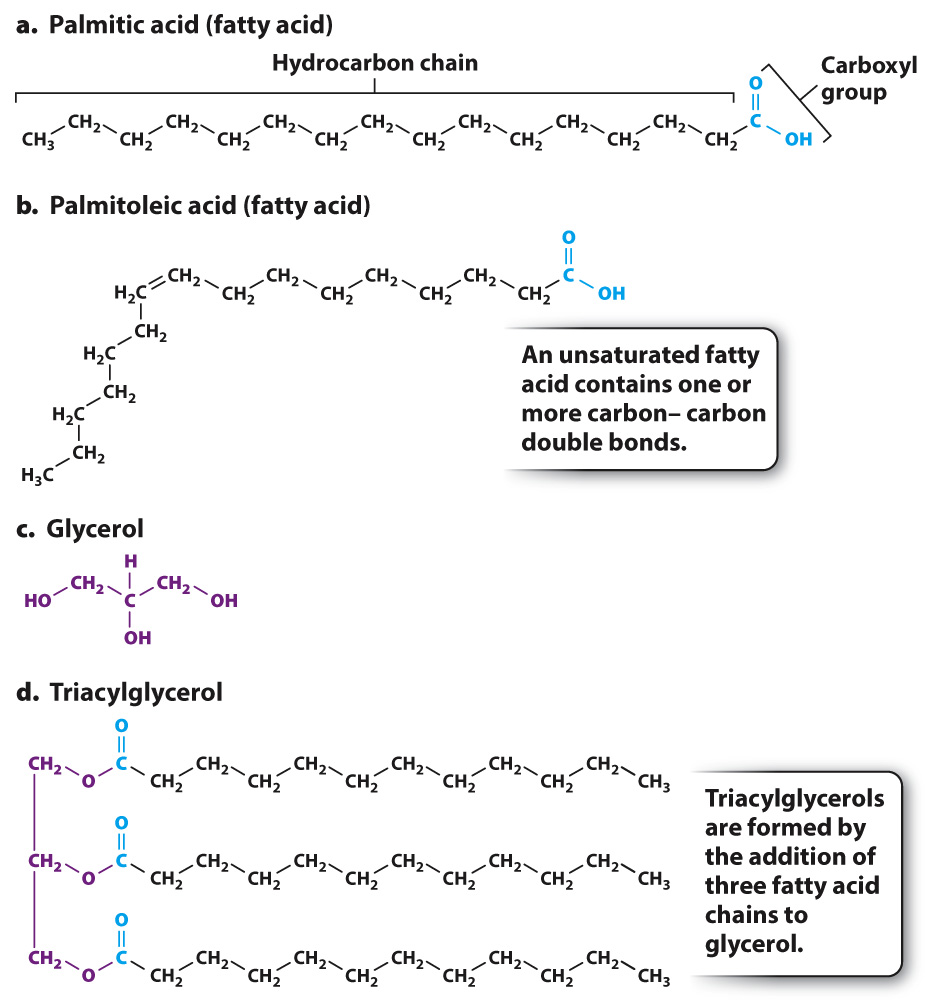
Fatty acids differ in the length of their hydrocarbon chain—
Some fatty acids have one or more carbon–
Triacylglycerols can contain different types of fatty acids attached to the glycerol backbone. The hydrocarbon chains of fatty acids do not contain polar covalent bonds like those in a water molecule. Their electrons are distributed uniformly over the whole molecule and thus these molecules are uncharged. As a consequence, triacylglycerols are all extremely hydrophobic and, therefore, form oil droplets inside the cell. Triacylglycerols are an efficient form of energy storage because, by excluding water molecules, a large number can be packed into a small volume.
Although fatty acid molecules are uncharged, the constant motion of electrons leads to regions of slight positive and slight negative charges (Fig. 2.26). These charges in turn attract or repel electrons in neighboring molecules, setting up areas of positive and negative charge in those molecules as well. The molecules are temporarily polarized molecules. Such molecules weakly bind to one another because of the attraction of opposite charges. These interactions are known as van der Waals forces. The van der Waals forces come into play only when atoms are sufficiently close to one another, and they are weaker than hydrogen bonds, but many van der Waals forces acting together help to stabilize molecules.
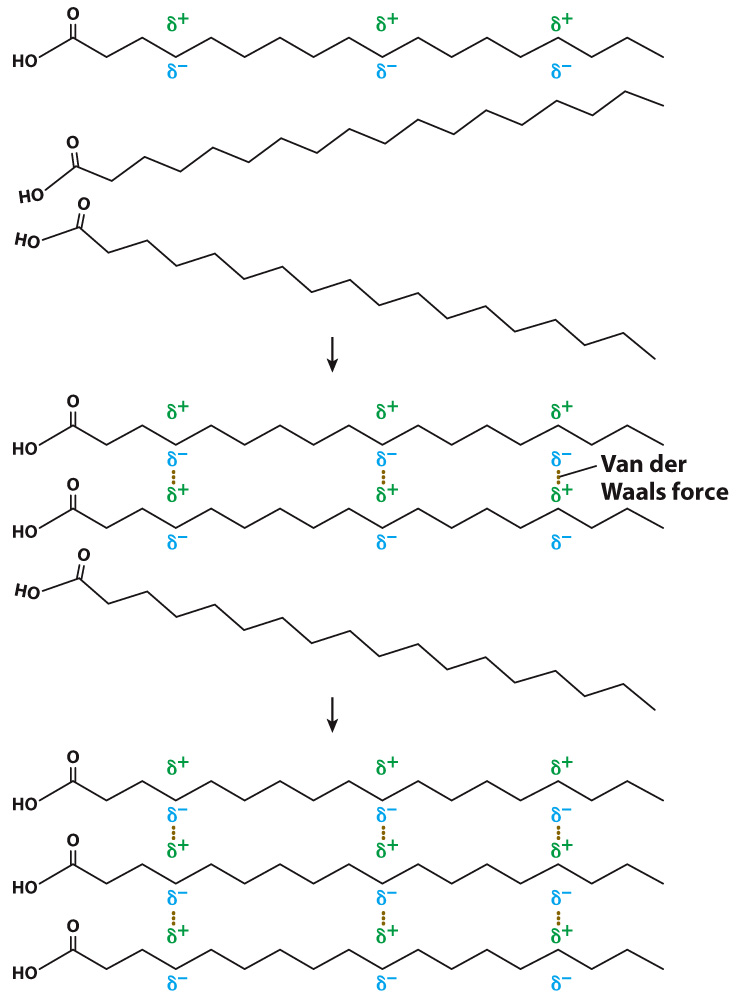
Because of van der Waals forces, the melting points of fatty acids depend on their length and level of saturation. As the length of the hydrocarbon chains increases, the number of van der Waals interactions between the chains also increases. The melting temperature increases because more energy is needed to break the greater number of van der Waals interactions. Kinks introduced by double bonds reduce the tightness of the molecular packing and thus the number of intermolecular interactions. As a result, the melting temperature is lower. Therefore, an unsaturated fatty acid has a lower melting point than a saturated fatty acid of the same length. Animal fats such as butter are composed of triacylglycerols with saturated fatty acids and are solid at room temperature, whereas plant fats and fish oils are composed of triacylglycerols with unsaturated fatty acids and are liquid at room temperature.
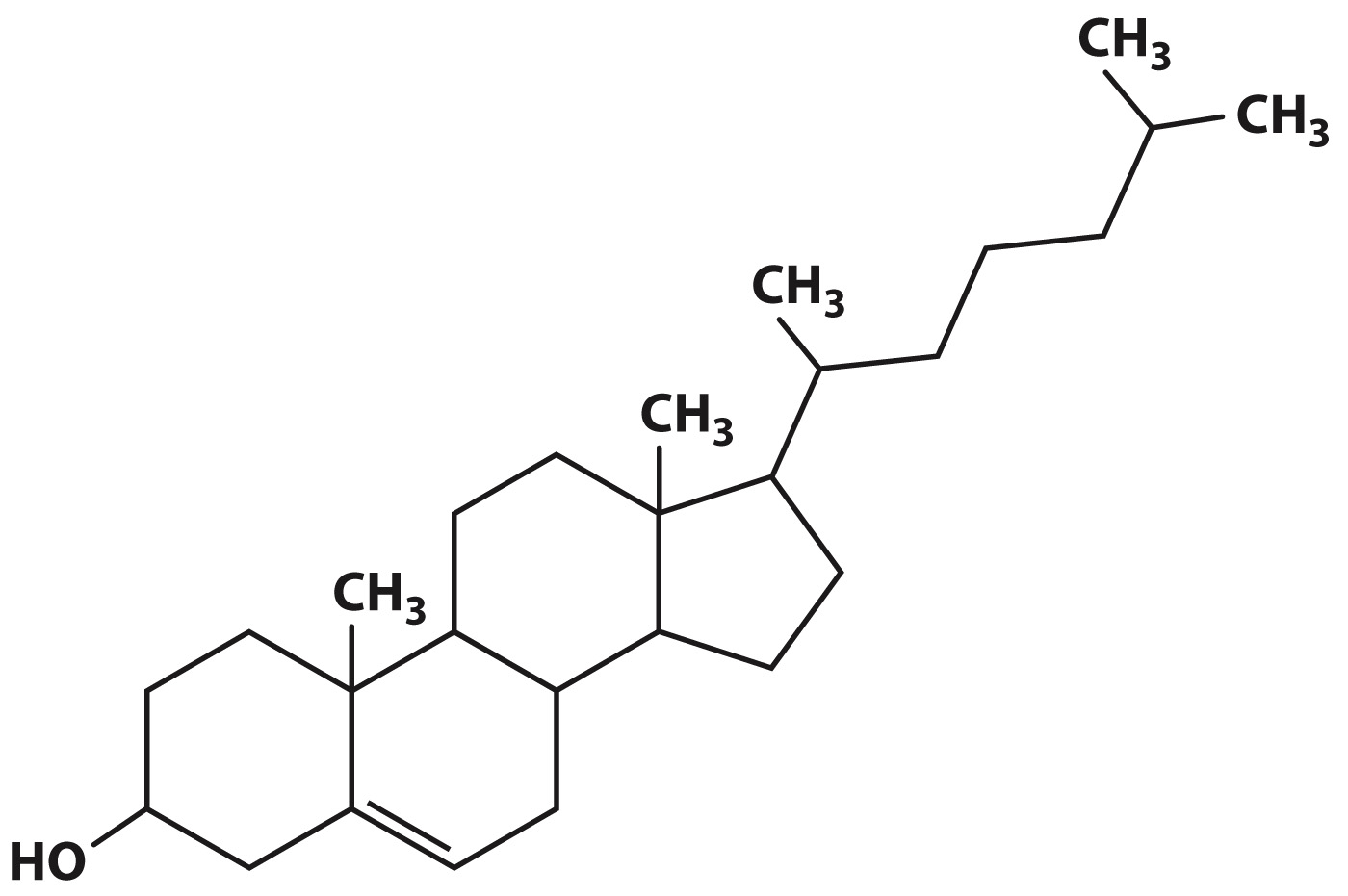
Steroids such as cholesterol are a second type of lipid (Fig. 2.27). Like other steroids, cholesterol has a core composed of 20 carbon atoms bonded to form four fused rings, and it is hydrophobic. Cholesterol is a component of animal cell membranes (Chapter 5) and serves as a precursor for the synthesis of steroid hormones such as estrogen and testosterone (Chapter 38).
Phospholipids are a third type of lipid. They are a major component of the cell membrane and are described in Chapter 5.