An ionic bond forms between oppositely charged ions.
In water, the difference in electronegativity between the oxygen and hydrogen atoms leads to unequal sharing of electrons. In more extreme cases, when an atom of very high electronegativity is paired with an atom of very low electronegativity, the difference in electronegativity is so great that the electronegative atom “steals” the electron from its less electronegative partner. In this case, the atom with the extra electron has a negative charge and is a negative ion. The atom that has lost an electron has a positive charge and is a positive ion. The two ions are not covalently bound, but because opposite charges attract they associate with each other in what is called an ionic bond. An example of a compound formed by the attraction of a positive ion and a negative ion is table salt, or sodium chloride (NaCl) (Fig. 2.8a).
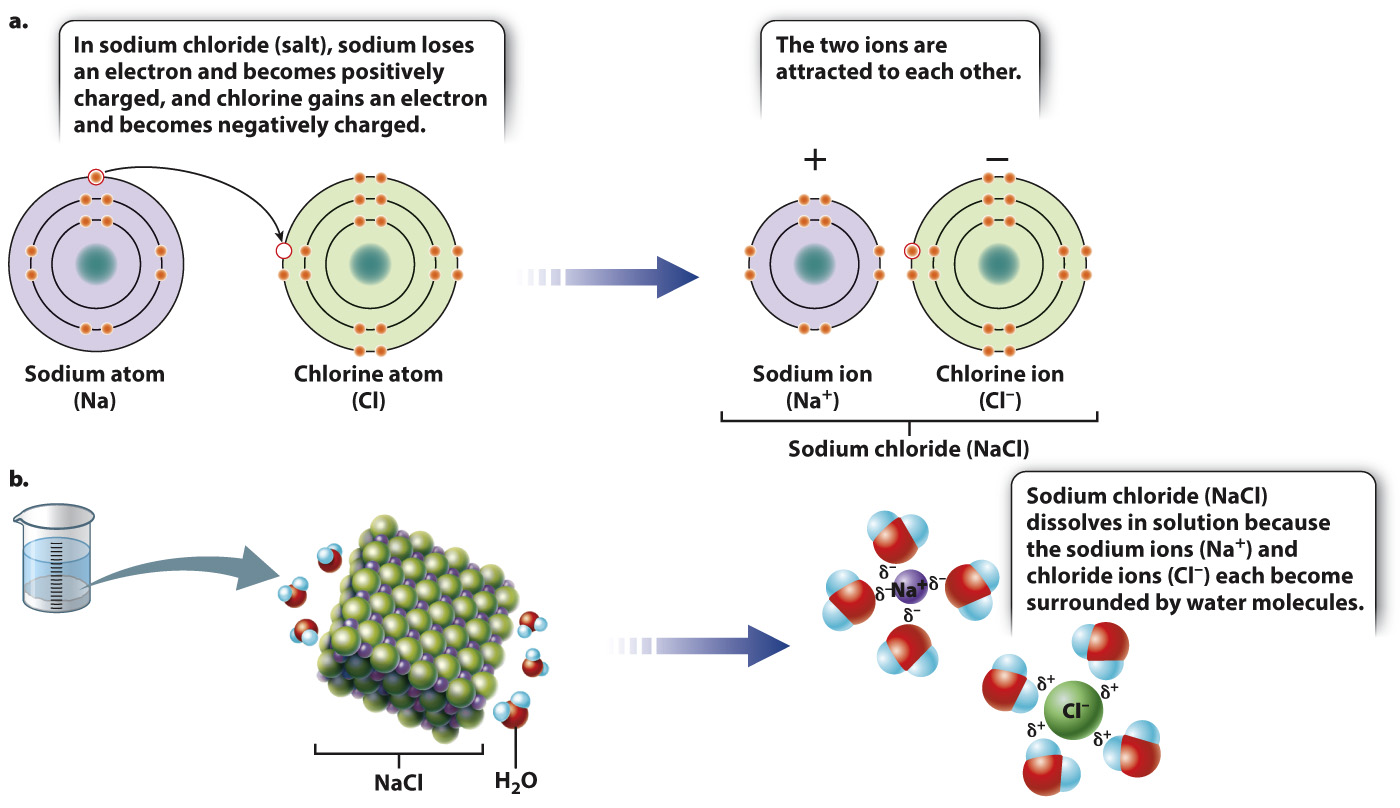
When sodium chloride is placed in water, the salt dissolves to form sodium ions (written as “Na+”) that have lost an electron and so are positively charged, and chloride ions (Cl–) that have gained an electron and so are negatively charged. In solution, the two ions are pulled apart and become surrounded by water molecules: The negatively charged ends of water molecules are attracted to the positively charged sodium ion, and the positively charged ends of other water molecules are attracted to the negatively charged chloride ion (Fig. 2.8b). Only as the water evaporates do the concentrations of Na+ and Cl- increase to the point where the ions join and precipitate as salt crystals.