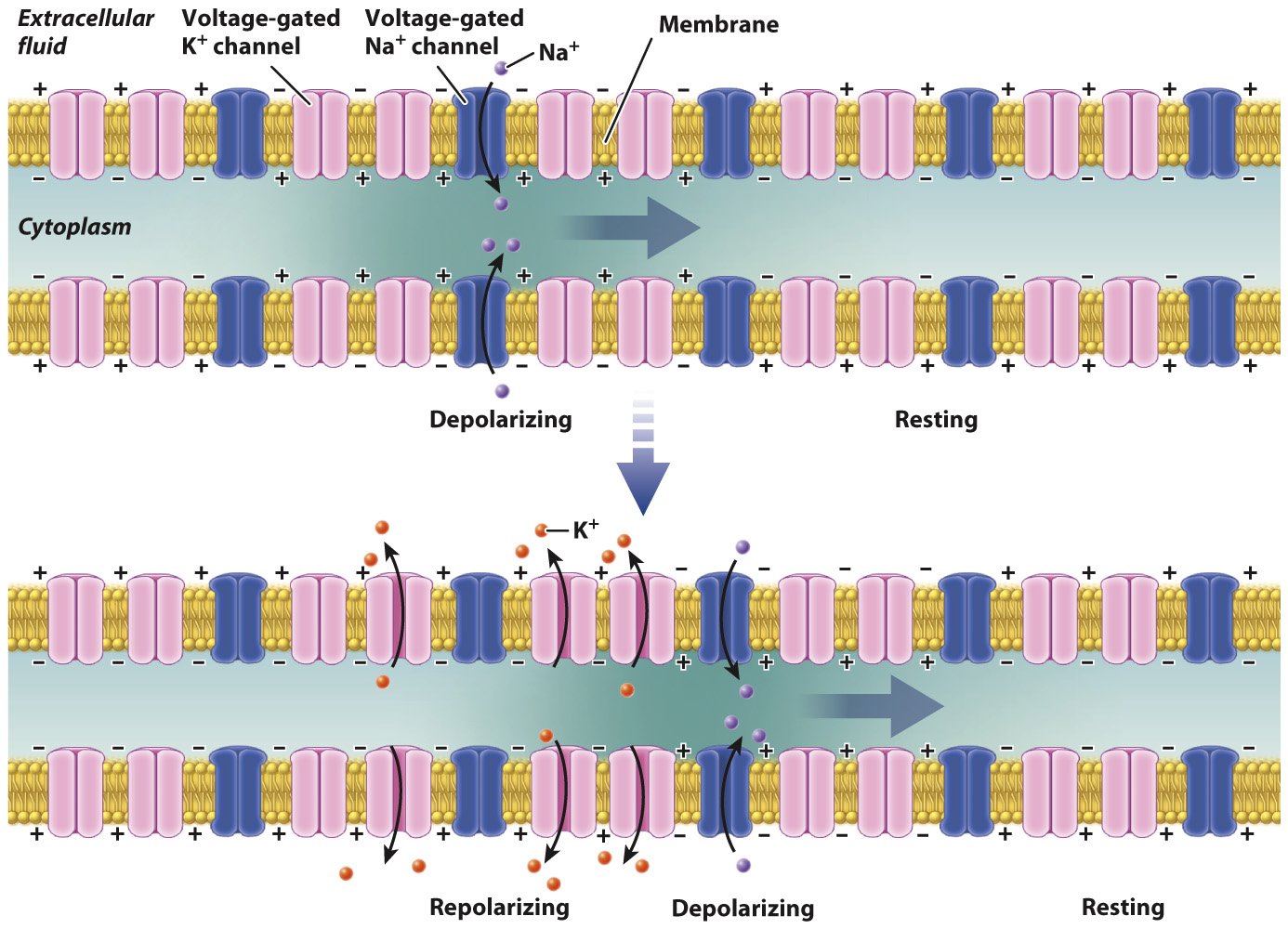
Neurons propagate action potentials along their axons by sequentially opening and closing adjacent Na+ and K+ ion channels.
Once initiated, an action potential propagates along the axon (Fig. 35.9). The local membrane depolarization initiated at the axon hillock triggers the opening of neighboring voltage-
The conduction speed of action potentials is limited by the neuron’s membrane properties and the width of its axon. Yet fast transmission speeds are essential for animals to respond quickly to stimuli. For example, when you mistakenly touch something very hot, your fingers sense and relay this information rapidly to ensure that you withdraw your hand quickly and avoid worse injury.
The neurons of vertebrate animals have an adaptation that lets them respond rapidly to stimuli in their environment. Glial cells form lipid-
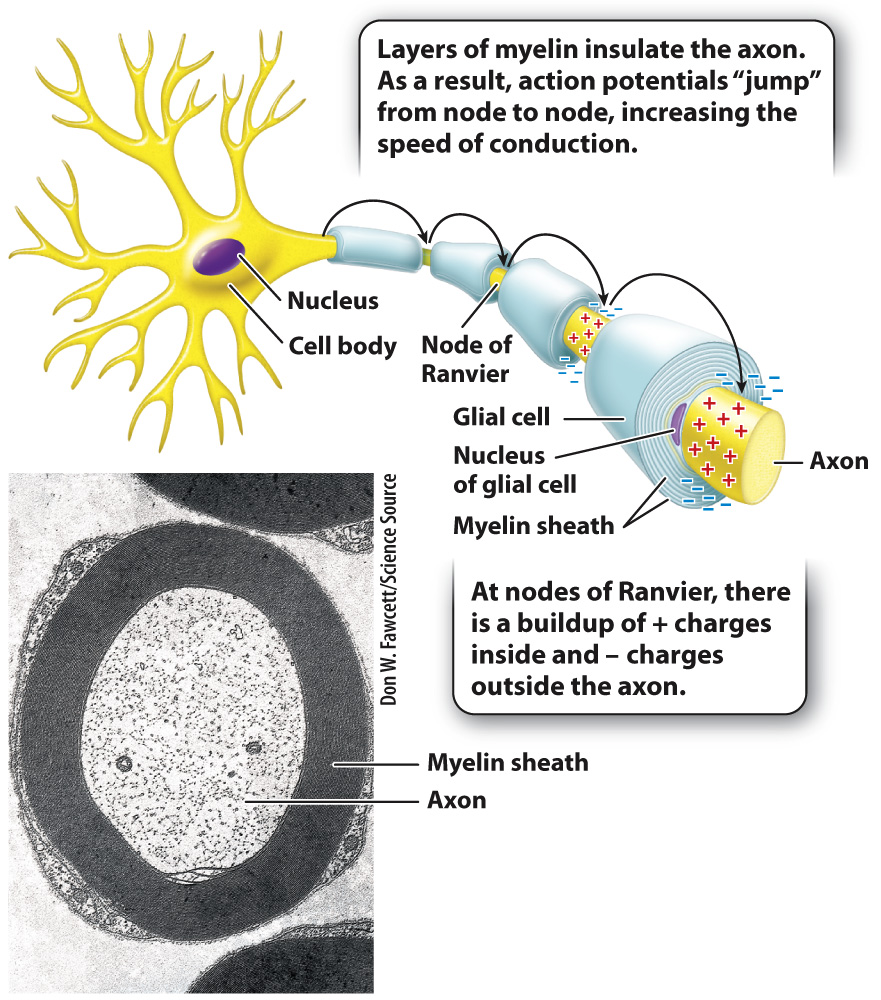
At regular intervals, the axon membrane is exposed at sites called nodes of Ranvier that lie between adjacent segments wrapped with myelin (Fig. 35.10). Voltage-
Quick Check 2 Multiple sclerosis is a disease in which the immune system attacks and destroys the myelin sheath surrounding nerve cells. What effect would you expect the loss of myelin to have on the speed of nerve impulses?
Quick Check 2 Answer
Nerve impulses are slowed or completely stopped in response to loss of myelin surrounding nerve cells.
The British neurophysiologists Alan Hodgkin and Andrew Huxley first worked out the electrical properties of the axon plasma membrane by studying the giant axon in squid that stimulates contractions of their body muscles for swimming (Fig. 35.11). For this work, they shared the 1963 Nobel Prize in Physiology or Medicine along with John Eccles, who worked out the membrane properties of synapses, which we explore next.
HOW DO WE KNOW?
FIG. 35.11
What is the resting membrane potential? How does electrical activity change during an action potential?
BACKGROUND In order to record the voltage of the inside of a nerve cell relative to the outside, a small electrical recording device called a microelectrode can be inserted into a neuron. The technique is more easily performed on large cells, such as the squid giant axon. This axon, as its name suggests, is quite large, measuring 0.5 mm in diameter (Fig. 35.11a). The large diameter of the axon allows electrical signals to be propagated to the muscles very quickly. Vertebrate neurons are much smaller; they rely on myelin sheaths rather than large size for rapid conduction of electrical signals.
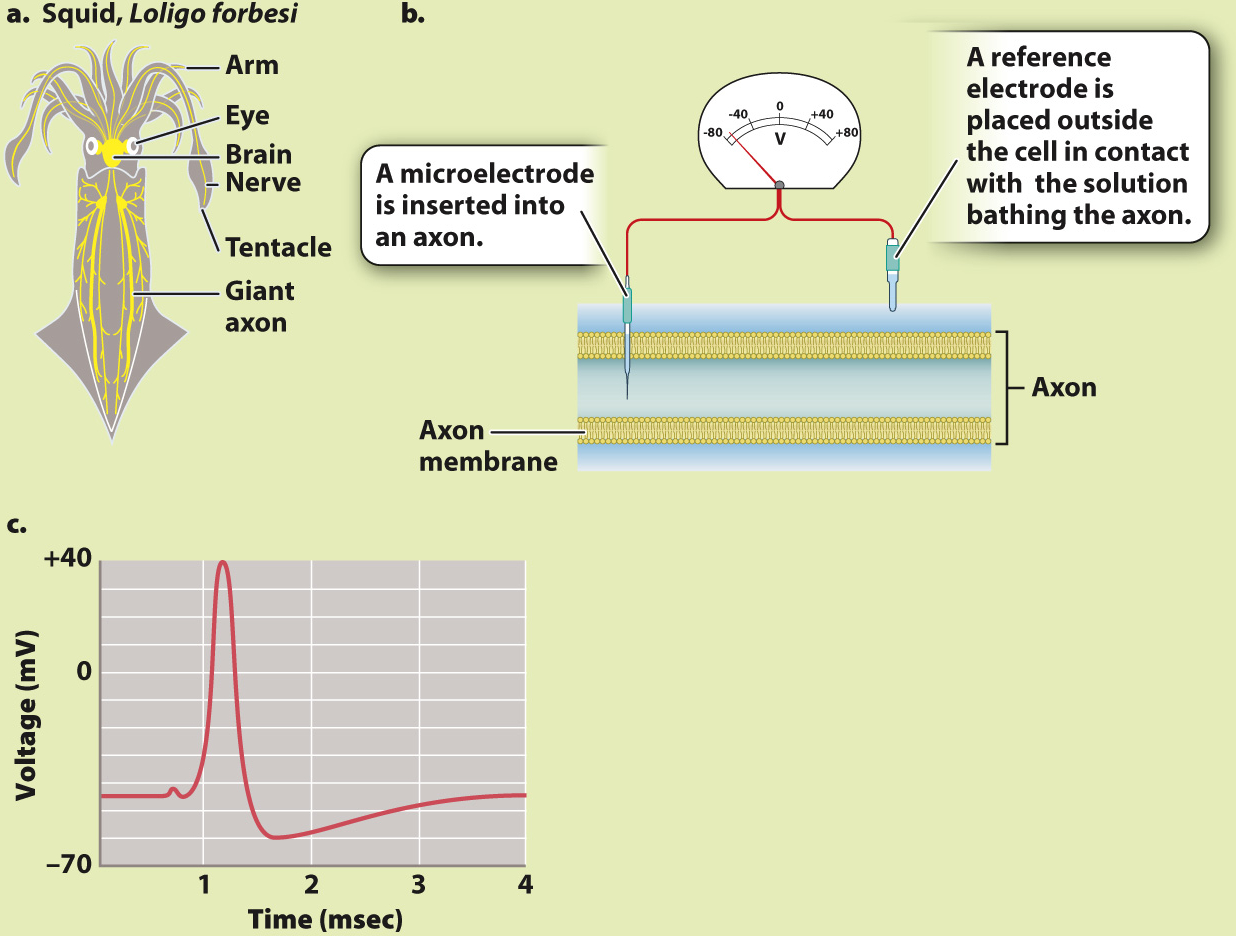
EXPERIMENT In 1939, two British neurophysiologists, Alan Hodgkin and Andrew Huxley, inserted a microelectrode into a squid giant axon and placed a reference electrode on the outside (Fig. 35.11b). They then used a separate set of electrodes (not shown) to depolarize the cell to threshold, triggering an action potential.
RESULTS Fig. 35.11c is a trace from Hodgkin and Huxley’s 1939 paper showing the resting membrane potential and the course of an action potential recorded by the electrode inside a giant squid axon. Note that the resting potential (–45 mV in squid) is negative on the inside of the axon relative to the outside, and that the action potential is a rapid spike in potential, with the inside of the cell quickly becoming positive, then negative again. The large size of the spike was a surprise.
FOLLOW-
SOURCES Hodgkin, A. L., and A. F. Huxley. 1939. “Action Potentials Recorded from Inside a Nerve Fibre.” Nature 144:710–