Muscles contract by the sliding of myosin and actin filaments.
Using high-
The banding patterns of sarcomeres revealed the extent of overlap between actin and myosin filaments. When myofibrils contracted to short lengths, the sarcomeres were observed to have increased actin–
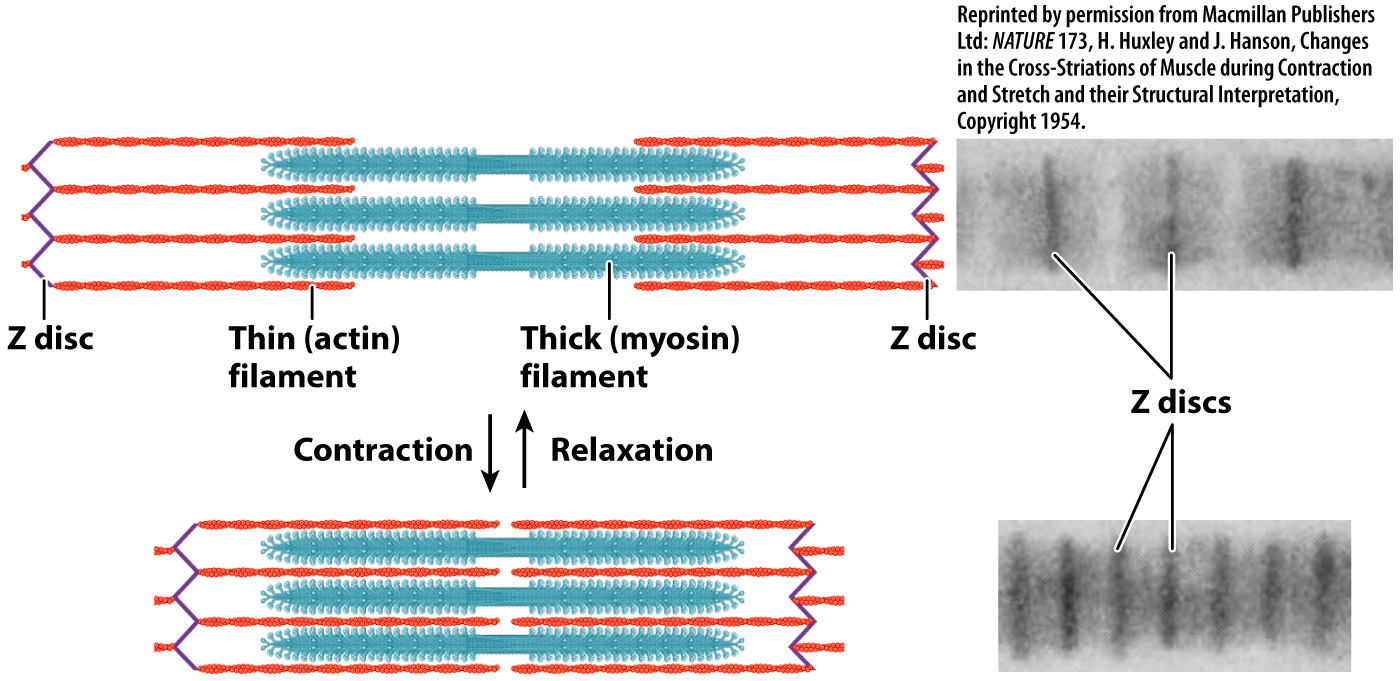
A muscle’s ability to generate force and change length is largely determined by the properties of its sarcomeres. Whereas sarcomere length is quite uniform in vertebrate animals (about 2.3 µm at rest), it is variable in invertebrate animals (ranging from as short as 1.3 µm to as long as 43 µm). Longer sarcomeres allow a greater degree of shortening. Thus, length changes are more limited in vertebrate muscles compared to those in some invertebrate muscles with long sarcomeres. For example, when the sarcomeres in an octopus tentacle contract, they can produce large motions of the tentacle. However, because vertebrates have shorter sarcomeres than an octopus, vertebrate muscles can shorten to produce movements more quickly.
Interactions between the myosin and actin filaments are what cause a muscle fiber to shorten and produce force. Along the myosin filament, one of the two heads of a myosin molecule at a given point in time binds to actin at a specific site to form cross-
The cross-
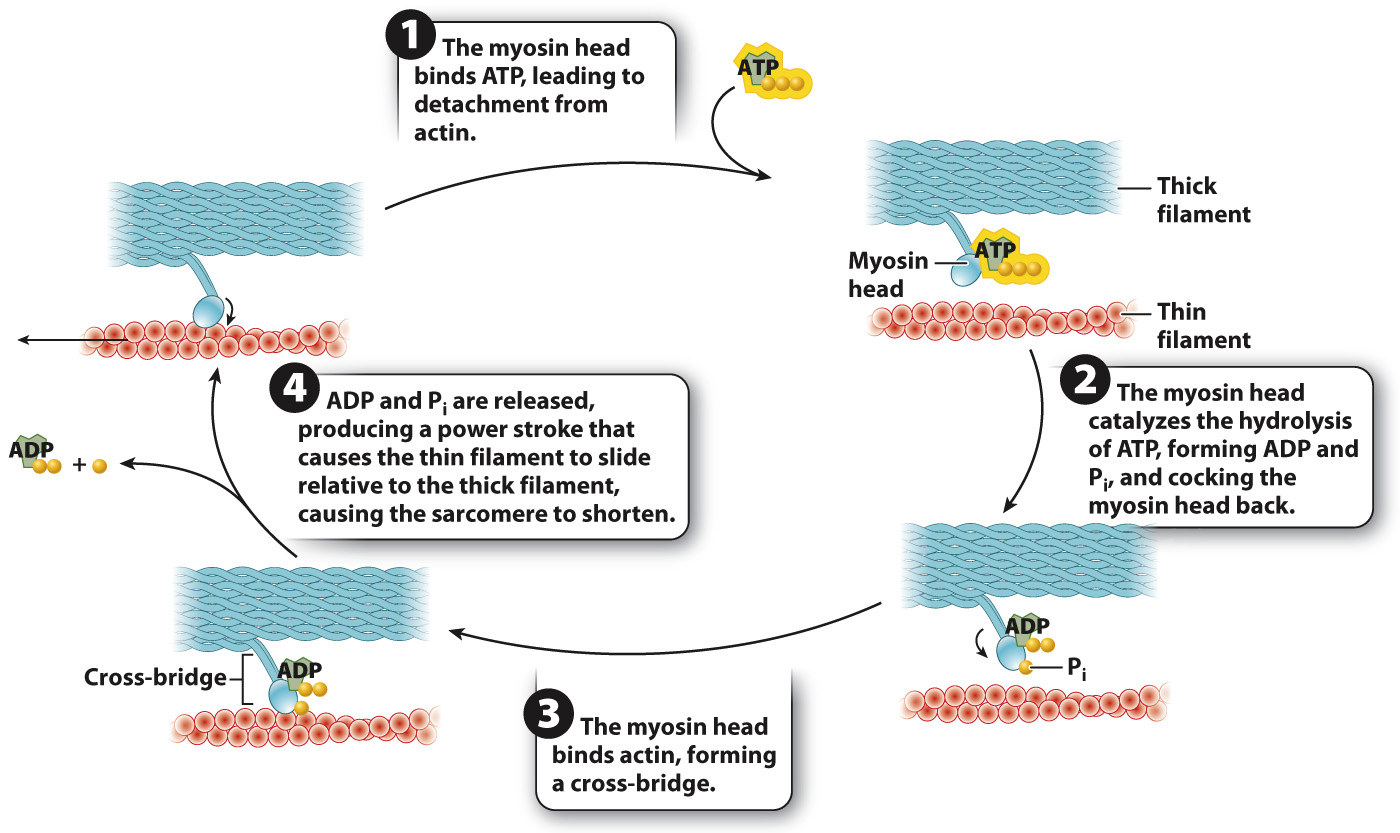
The myosin head binds ATP. Binding of ATP allows the myosin head to detach from actin and readies it for attachment to actin.
The myosin head hydrolyzes ATP to ADP and inorganic phosphate (Pi). Hydrolysis of ATP results in a conformational change in which the myosin head is cocked back. ADP and Pi remain bound to the myosin head. Because ADP and Pi are bound rather than released, the myosin head is in a high-
energy state. The myosin head then binds actin, forming a cross-
bridge. When the myosin head binds actin, the myosin head releases ADP and Pi. The result is another conformational change in the myosin head, called the power stroke. During the power stroke, the myosin head pivots forward and generates a force, causing the myosin and actin filaments to slide relative to each other over a distance of approximately 7 nm. The power stroke pulls the actin filaments toward the sarcomere midline.
The myosin head then binds a new ATP molecule (step 1 again), allowing it to detach from actin, and the cycle repeats. After detachment, the myosin head again hydrolyzes ATP, allowing it to return to its original conformation and bind to a new site farther along the actin filament. With the release of ADP and Pi, the myosin head undergoes another cycle of force generation and movement.
Individual muscle contractions are the result of many successive cycles of cross-
Because each thick filament can interact with as many as six actin filaments, at any one time numerous cross-
Quick Check 1 When an animal dies, its limbs and body become stiff because its muscles go into rigor mortis (literally, rigor mortis means “stiffness of death”). Why would the loss of ATP following death cause this to happen?
Quick Check 1 Answer
Without newly synthesized ATP, myosin cross-