Many factors affect hemoglobin–oxygen binding.
Obtaining enough O2 is a special challenge in some circumstances. How does a mammalian fetus in its mother’s uterus take up O2 from its mother’s blood? How do animals that live at high altitude obtain O2 under conditions of low pO2? In each case, the species adapts by evolving forms of hemoglobin with higher binding affinity.
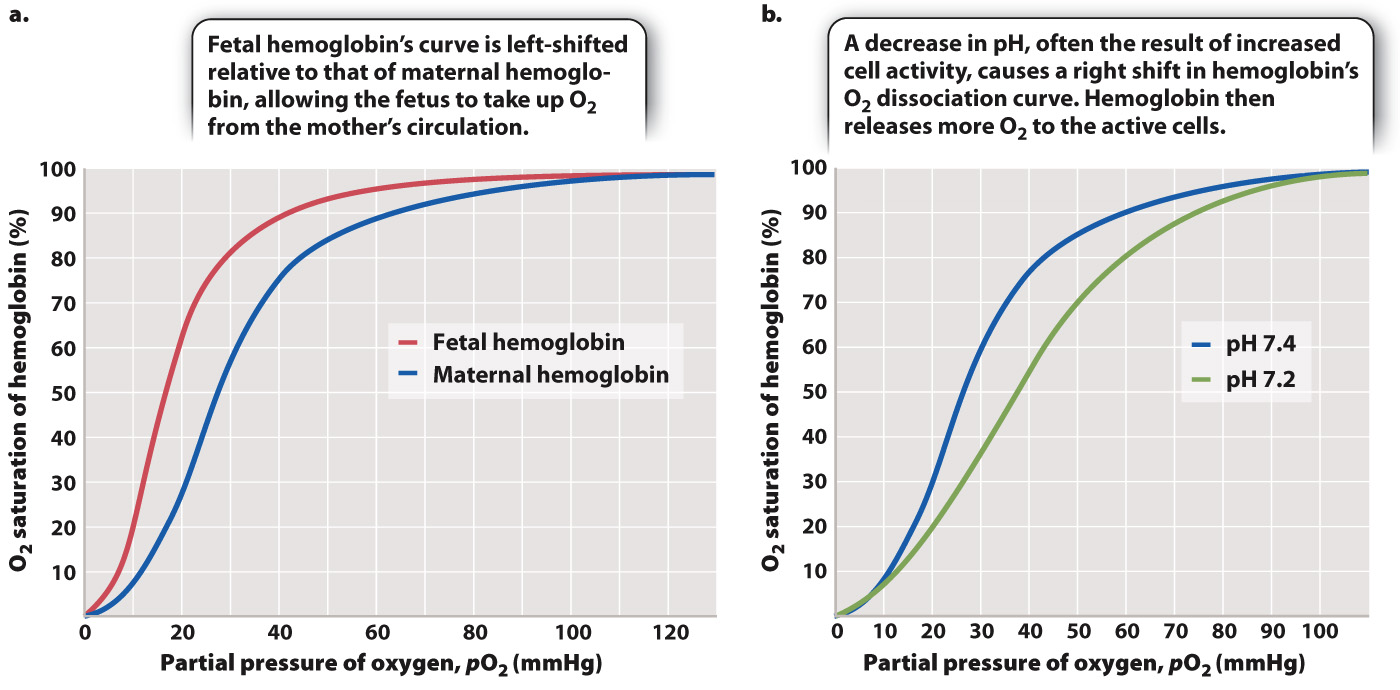
In mammals, the mother’s respiratory and cardiovascular systems must provide the fetus with O2 and remove CO2, as well as supply nutrients and remove waste. Maternal and fetal blood do not mix. Rather, they exchange gases across the placenta. Yet the O2 concentration gradient at this exchange point does not strongly favor the movement of O2 from the mother’s blood to the fetal blood. Fetal mammals solve the problem of extracting O2 from their mother’s hemoglobin by producing a form of hemoglobin before birth that has a higher affinity for O2 than their mother’s hemoglobin. The O2 dissociation curve for fetal hemoglobin is shifted to the left of the curve for maternal hemoglobin (Fig. 39.15a), allowing the fetal hemoglobin to extract O2 from its mother’s circulation. At birth, when the newborn begins to breathe, its red blood cells rapidly shift to producing the adult form of hemoglobin, maintaining this form throughout life.
Many animals live at high altitude. For example, birds have been observed flying over the highest peaks (taller than 9000 m) despite the fact that air at this altitude has less than one-
Tissue and blood pH also have an important physiological effect on the O2 affinity of hemoglobin. When pH falls during exercise (that is, when H+ concentration increases), the affinity of hemoglobin for O2 decreases, resulting in a rightward shift in the O2 dissociation curve (Fig. 39.15b). This phenomenon is called the Bohr effect after Christian Bohr, the Danish physiologist who first described it in 1904. Decreases in pH occur when CO2 is released from metabolizing cells, or when an inadequate supply of O2 leads to the production of lactic acid (Chapter 7). Because hemoglobin’s affinity for O2 is reduced, more O2 is released and supplied to the cells for aerobic ATP synthesis.
Carbon dioxide also reacts with the amine (NH2) groups of hemoglobin, reducing hemoglobin’s affinity for O2. Thus, when released from respiring tissues, CO2 promotes increased O2 delivery both through its direct effect on hemoglobin and its contribution to a decrease in blood pH by the Bohr effect. While the production of CO2 promotes O2 release at the tissues, its elimination at the lung increases hemoglobin’s affinity for O2, enhancing O2 uptake.
Quick Check 4 Sickle-
Quick Check 4 Answer
Sickle-