Diffusion governs gas exchange over short distances.
Single-
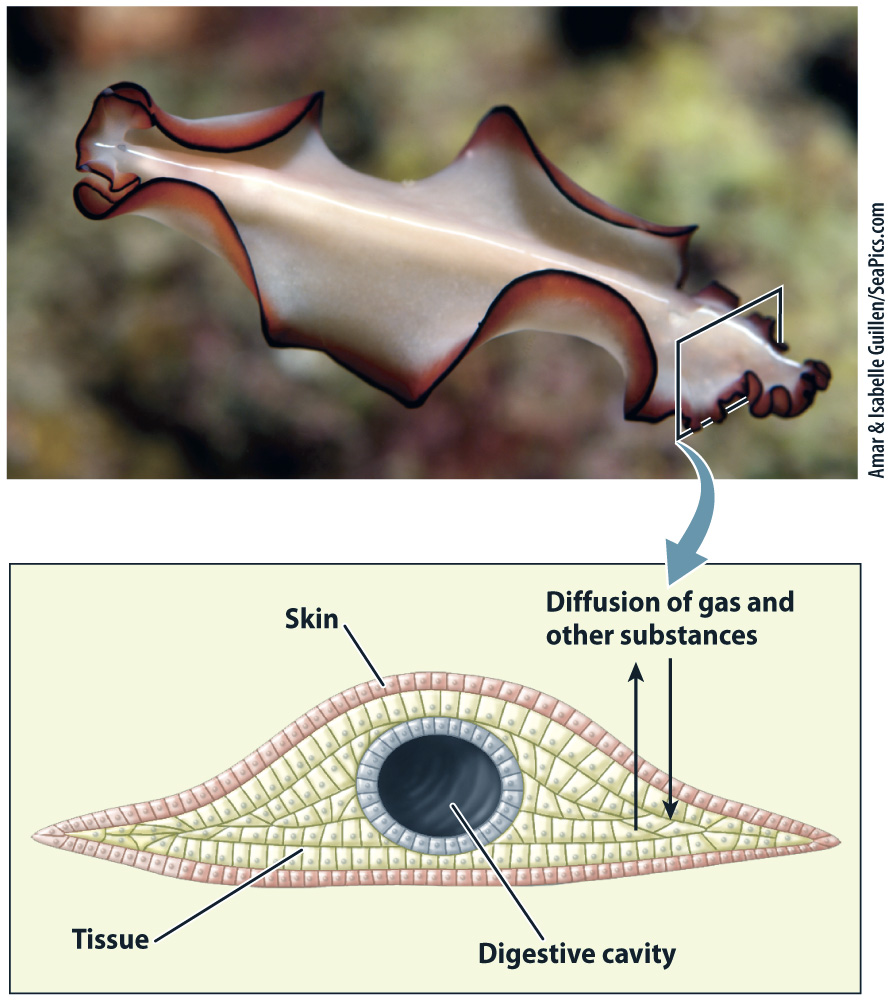
Given a large enough surface, diffusion is an extremely effective way to exchange gases and other substances over short distances between two compartments, for example between the lungs and the blood vessels supplying the lungs. The rate of diffusion is directly proportional to the surface area over which exchange occurs and to the concentration difference, and inversely proportional to the distance over which the molecules move (or the thickness of the barrier). Diffusion is generally ineffective over distances exceeding about 100 µm, or 0.1 mm. Consequently, respiratory and circulatory structures have large surface areas and thin interfaces.
Because effective transport by diffusion is limited to short distances, some of the earliest and simplest invertebrate animals are composed of thin sheets of cells with a large surface area. For example, sponges and sea anemones have a thin body with a central cavity through which water circulates, and flatworms have a flattened body surface (Fig. 39.1).
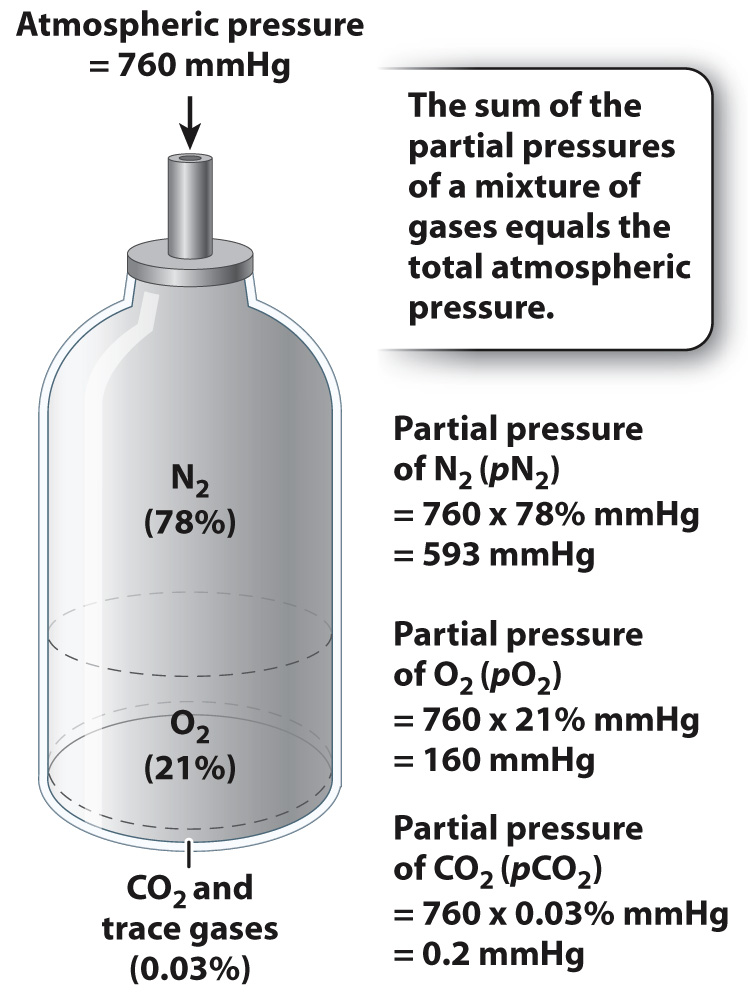
Net movement of a substance results from diffusion when there is a concentration difference between two regions. When considering the diffusion of a gas, however, we usually refer to its partial pressure rather than its concentration. The partial pressure (p) of a gas is defined as its fractional concentration relative to other gases present, multiplied by the overall (that is, the atmospheric) pressure (Fig. 39.2). Pressure is measured in units of millimeters of mercury (mmHg). Oxygen makes up approximately 21% of the gases in air (nitrogen contributes about 78%, carbon dioxide about 0.03%, and the remainder are trace gases). At sea level, atmospheric pressure is approximately 760 mmHg, which means that O2 in the atmosphere exerts a partial pressure (pO2) of about 160 mmHg (0.21 × 760). For O2 to diffuse from the air into cells, the partial pressure of O2 inside the cells must be lower than the partial pressure of O2 in the atmosphere.
Quick Check 1 What two structural features favor diffusion?
Quick Check 1 Answer
Diffusion is increased by a large surface area for exchange and a short diffusion distance.