RNA is a polymer of nucleotides in which the 5-carbon sugar is ribose.
RNA is a polymer of nucleotides linked by phosphodiester bonds similar to those in DNA (see Fig. 3.7). Each RNA strand therefore has a polarity determined by which end of the chain carries the 3′ hydroxyl (–OH) and which end carries the 5′ phosphate. There are a number of important differences that distinguish RNA from DNA, however (Fig. 3.13). First, the sugar in RNA is ribose, which carries a hydroxyl group on the 2′ carbon (highlighted in pink in Fig. 3.13a). Hydroxyls are reactive functional groups, so the additional hydroxyl group on ribose in part explains why RNA is a less stable molecule than DNA. Second, the base uracil in RNA replaces thymine in DNA (Fig. 3.13b). The groups that participate in hydrogen bonding (highlighted in pink in Fig. 3.13b) are identical so that uracil pairs with adenine (U–A) just as thymine pairs with adenine (T–A). Third, while the 5′ end of a DNA strand is typically a monophosphate, the 5′ end of an RNA molecule is typically a triphosphate.
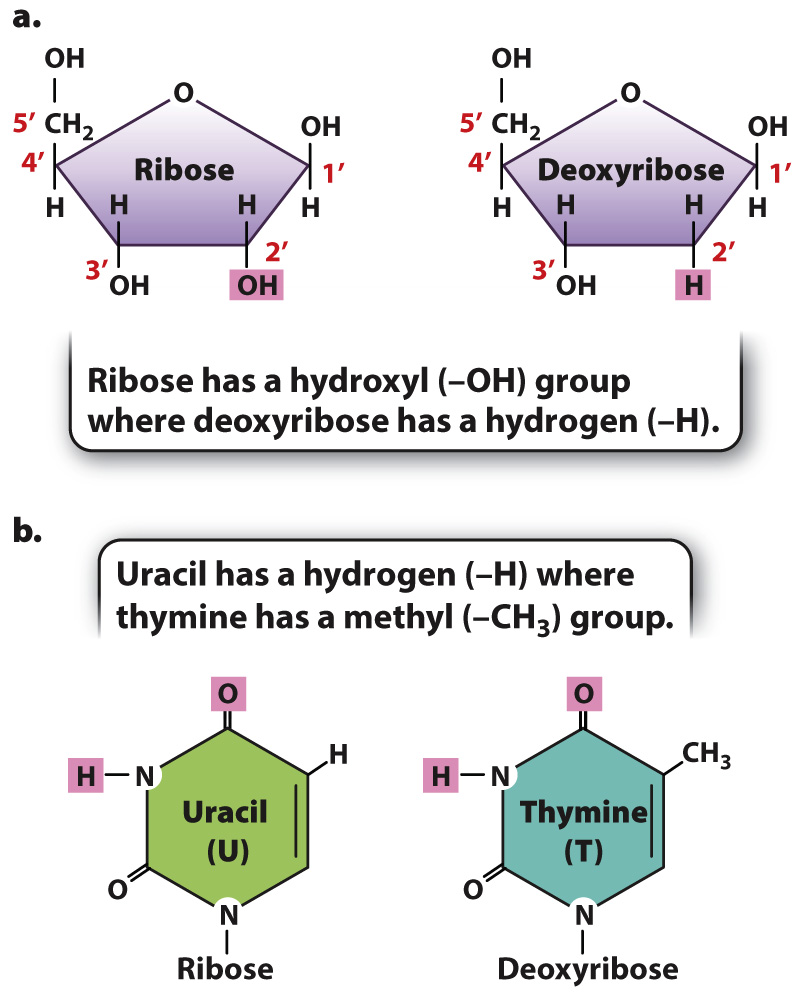
Two other features that distinguish RNA from DNA are physical rather than chemical. One is that RNA molecules are usually much shorter than DNA molecules. A typical RNA molecule used in protein synthesis consists of a few thousand nucleotides, whereas a typical DNA molecule consists of millions or tens of millions of nucleotides. The other major distinction is that most RNA molecules in the cell are single stranded, whereas DNA molecules, as we saw, are double stranded. Single-