Molecular techniques provide new ways of testing the role of genes in behavior.
Molecular biology is changing the way we approach behavioral genetics. Some studies have identified complex behaviors that are strongly influenced by a single gene. One well-
In Drosophila, there are two different alleles of the foraging (for) gene—
Both forms are present in natural populations (Fig. 45.7b); typically, 70% are rovers, 30% are sitters. That both strategies are present in populations suggests that both are adaptive because, if one strategy were markedly inferior, we would expect natural selection to eliminate it. Furthermore, studies have shown that rovers are selected for in crowded environments in which there is an advantage to seeking new food sources, and sitters are selected for in less crowded ones because they take maximum advantage of their current food source. In this case, variation at a single gene affects a complex behavior in fruit flies.
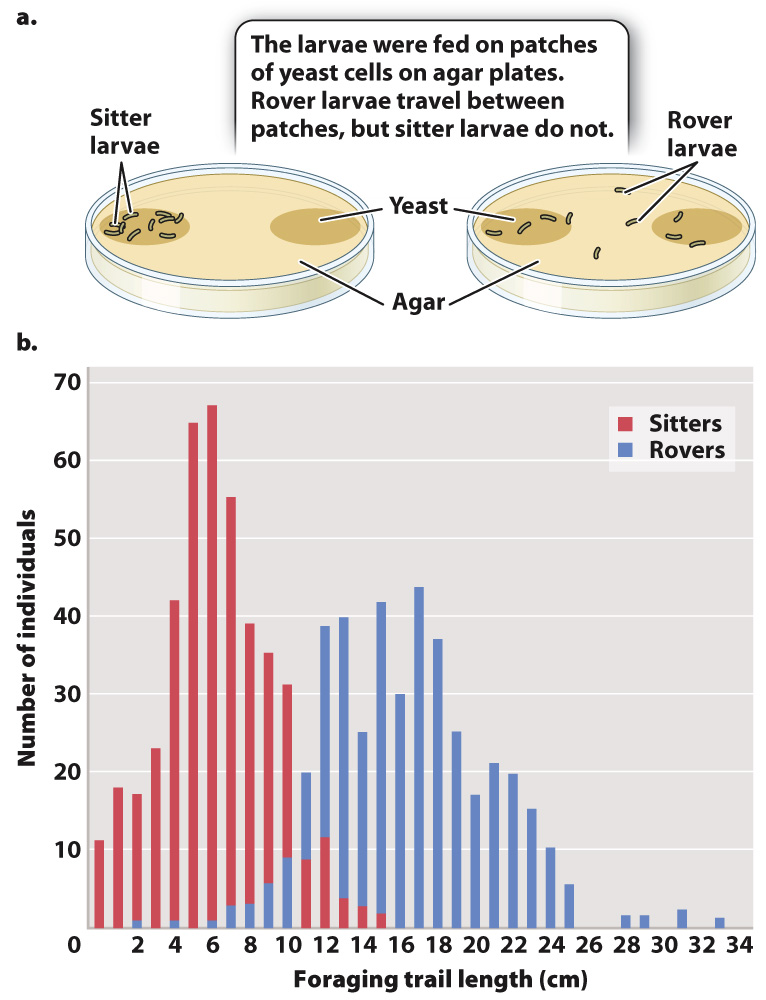
The foraging gene is present in other insects as well, including honeybees, suggesting that it has been evolutionary conserved. Honeybees with low levels of for expression in their brain tend to stay in the hive, whereas those with high levels of for expression are likely to be foragers (Fig. 45.8).
HOW DO WE KNOW?
FIG. 45.8
Can genes influence behavior?
BACKGROUND In fruit flies (Drosophila melanogaster), variation at the foraging (for) gene affects feeding behavior of larvae. Two alleles of the for gene—
HYPOTHESIS Foraging in honeybees is influenced by the expression of the for gene, and nurses have lower expression levels than foragers.
EXPERIMENT 1 Levels of for mRNA were measured in honeybee nurses and foragers.
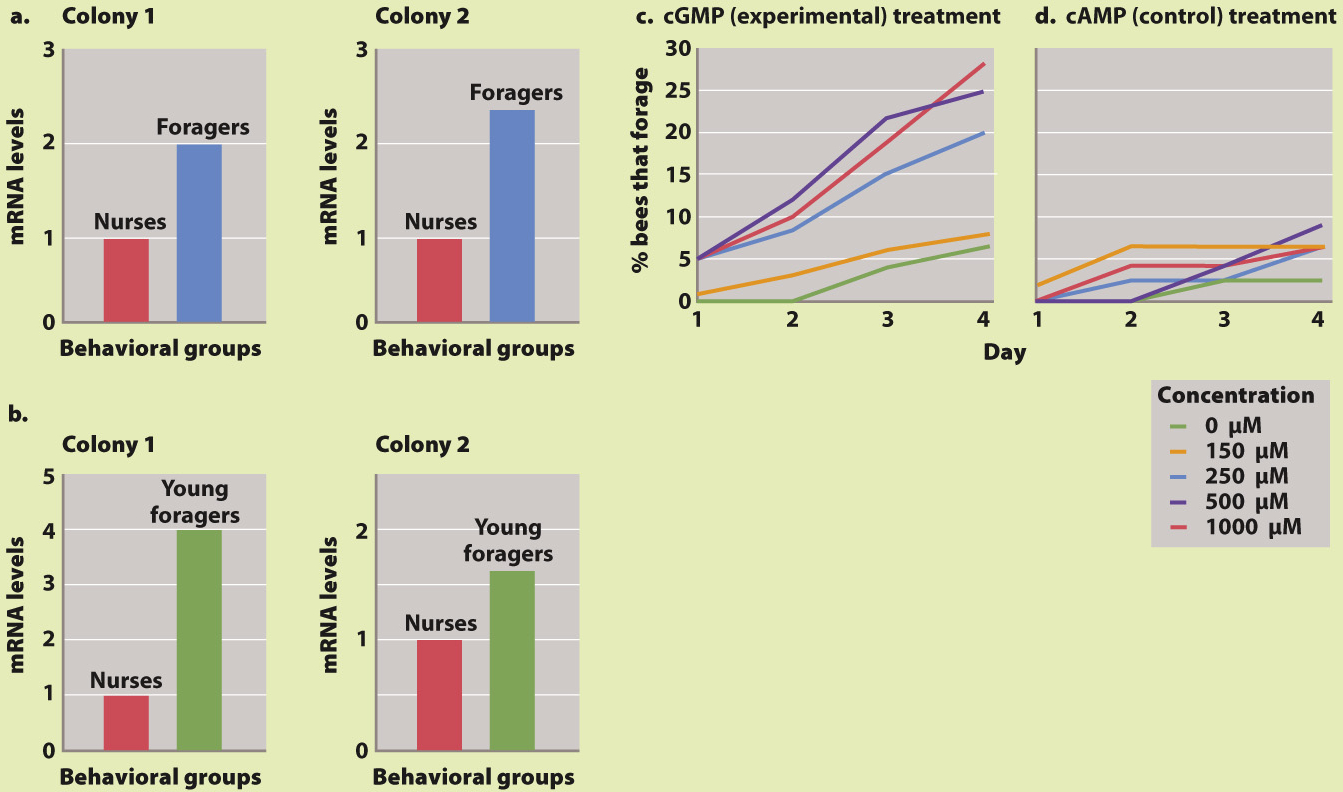
RESULTS Levels of for mRNA are significantly higher in foragers than in nurses (Fig. 45.8a). However, foragers are older than nurses, so it was unclear whether the increased expression in foragers compared with nurses is associated with differences in age or differences in behavior. Therefore, the experiment was repeated with honeybees that forage at the age at which bees are normally nurses. These young foragers also have higher for mRNA levels than do nurses (Fig. 45.8b).
EXPERIMENT 2 The for gene encodes a cGMP-
RESULTS Treatment with cGMP changed the behavior of nurses, causing them to forage (Fig. 45.8c). Furthermore, cGMP acted in a dose-
CONCLUSION The same gene that is involved in two different behavioral phenotypes in fruit flies (sitters versus rovers) is also involved in a developmental behavioral change in honeybees (nurses versus foragers). This finding suggests that a gene can have related but different functions in different organisms.
FOLLOW-
SOURCE Ben-

Studies of the North American vole, in the genus Microtus, have also revealed how genes influence behavior (Fig. 45.9). The prairie vole is monogamous: Couples pair bond for life, meaning that they mate only with each other. By contrast, the prairie vole’s close relative, the montane vole, is promiscuous: A male mates and moves on, and a female over her lifetime produces litters by several different males. What differences underlie these different behaviors?
Hormones and their receptors provide an answer. In all mammals, antidiuretic hormone (ADH) controls the concentration of urine (Chapter 41). The receptor for ADH is not only expressed in the kidney, but also in the brain, where it affects behavior. Both the monogamous prairie vole and the promiscuous montane vole produce ADH, but the hormone receptors in the two species are different. The American neuroscientist Thomas Insel analyzed the genes that encode the ADH receptor in the two vole species. He found a difference in the region of the gene that determines under what conditions and in what cells it is expressed. Because of this difference, the distribution of ADH receptors in the brain is different in the prairie vole and in the montane vole.
Does the difference in gene expression explain the different sexual behaviors of the two species? The answer seems to be yes. Insel and his colleague Larry Young inserted the prairie vole ADH receptor gene, complete with its regulatory region, into a laboratory mouse (a promiscuous species, like the montane vole). Interestingly, Insel and Young observed a marked change in the mouse’s behavior. Rather than mating with a female and then moving on to another mate, the transgenic male mouse pair bonded with the female. In short, the addition of a single gene affected a complex behavior such as pair bonding, making the otherwise promiscuous mouse more likely to pair bond.