CASE 8 BIODIVERSITY HOTSPOTS: RAIN FORESTS AND CORAL REEFS
How has global environmental change affected coral reefs around the world?

FIG. 49.11 The Great Barrier Reef. The world’s largest coral reef, lying off the coast of Australia, is visible from space.
Increasing atmospheric CO2 is not just influencing life on land. It is also having an observable impact on organisms in the oceans, not least on coral reefs that provide hotspots of biological diversity in the marine realm. The Great Barrier Reef is visible from space, its wave-washed necklace of limestone stretching more than 2000 km along the northeastern coast of Australia (Fig. 49.11). A global diversity hotspot, the reef’s 1500 fish species (a tenth of all known fish), more than 4000 species of clams and snails, and myriad other species prompt basic questions about ecology and diversity, many of which we have addressed in previous chapters. Lately, however, one question has come to dominate scientific discussion of the Great Barrier Reef: Will it exist a century from now?
Page 1078
Corals are the principal architects of the Great Barrier and most other reefs (Chapter 44), and so coral biology is the key to understanding both the reef’s unusual diversity and its vulnerability in the face of climate change. Most reef corals gain nutrition from unicellular algae that live within their tissues. The algal symbionts in reef corals provide food for their host, in return receiving nutrients, a means of waste disposal, and a stable environment. It is the efficient cycling of carbon, nitrogen, and phosphorus between corals and their symbionts that underpins the biological richness of coral reefs. Reef corals accomplish another noteworthy feat: They make skeletons of calcium carbonate (CaCO3) that through time build the reef’s three-dimensional framework.
The very processes that facilitate reef growth—biomineralization and microbial symbiosis—are exposing their Achilles’ heel in the 21st century. Today, on the Great Barrier Reef, throughout the Caribbean, and elsewhere, corals are dying. Bleaching, indicated by the white skeletons of dead corals, occurs when the symbiotic algae that feed the corals abandon their hosts, thereby sentencing them to death (Fig. 49.12). The details are still being investigated, but an increase in seawater temperature appears to signal the algal exodus. Increasing seawater temperature also facilitates the spread of infections that can kill coral.

FIG. 49.12 Coral bleaching. This coral shows a pattern of whitening that occurs when corals lose their algal symbionts.
A greater long-term threat, however, may come from increasing CO2 levels in the ocean. As noted in Chapter 25, not all the CO2 produced by humans during the past century has accumulated in the atmosphere: About one-third has been absorbed by the ocean. That’s good news for slowing climate change because it means there is less CO2 in the atmosphere to trap heat, but it is potentially bad news for marine habitats because CO2 dissolves in seawater to form carbonic acid (H2CO3) (Chapters 6 and 39). Unlike climate change, the effects of increasing CO2 on ocean pH are straightforward: Increasing the abundance of CO2 in the oceans causes the pH of seawater to go down, a phenomenon known as ocean acidification.
In the past 50 years, as the concentration of CO2 in the atmosphere has grown from about 315 ppm to about 400 ppm, the pH of surface oceans has correspondingly dropped by 0.1 unit (Fig. 49.13). That may not seem like a lot, but remember that the pH scale is logarithmic, and so the 0.1 unit drop represents an increase in hydrogen ions of about 30%. In turn, ocean acidification causes carbonate ions in seawater to decrease, making it more difficult for some marine algae and corals to build their CaCO3 skeletons (Fig. 49.14).
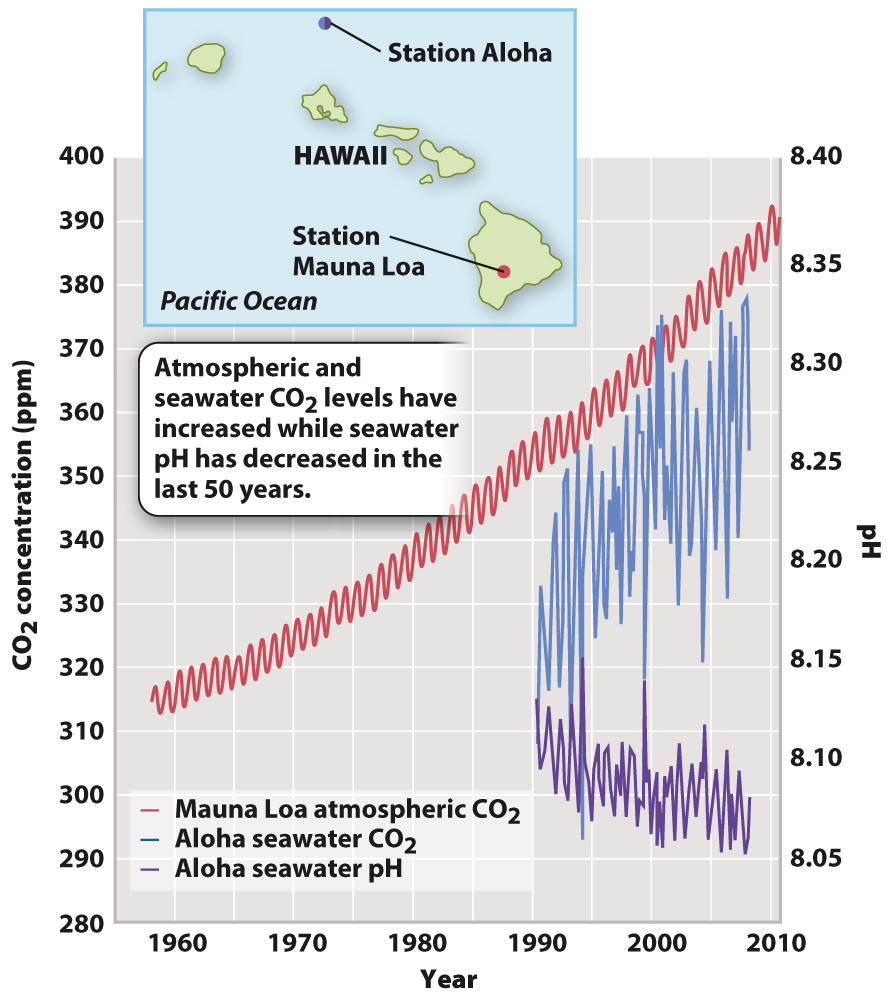
FIG. 49.13 Trends in atmospheric CO2 levels (red), seawater CO2 levels (blue), and seawater pH (purple) in the central Pacific Ocean. Data from: Doney, S.C. et al., Ocean Acidification: The Other CO2 Problem. Annual Review of Marine Science 1: 169–192.
Data from: Doney, S.C. et al., Ocean Acidification: The Other CO2 Problem. Annual Review of Marine Science 1: 169–192.
Page 1079
Page 1080
HOW DO WE KNOW?
What is the effect of increased atmospheric CO2 and reduced ocean pH on skeleton formation in marine algae?
BACKGROUND It is well established that atmospheric CO2 levels are increasing, which in turn decreases the pH of ocean water. In the late 1990s, experiments showed that a decrease in pH affected the ability of some marine organisms to build skeletons made of calcium carbonate (CaCO3). The German biologist Ulf Riebesell and his colleagues carried out experiments to investigate whether ocean acidification affects algae called coccolithophorids, which account for the majority of carbonate precipitation in the open ocean.
HYPOTHESIS From the effects of decreased pH on CaCO3 skeleton formation in other marine organisms, Riebesell hypothesized that increasing CO2 would interfere with skeleton formation in coccolithophorids.
EXPERIMENT Riebesell and his colleagues studied two species of coccolithophorids, Emiliania huxleyi and Gephyrocapsa oceanica. They grew both species in the laboratory under conditions of increasing CO2, from pre-industrial levels, to present-day levels, and then to levels predicted for the future. They measured pH and rates of calcification and used scanning electron microscopy to observe skeleton formation.
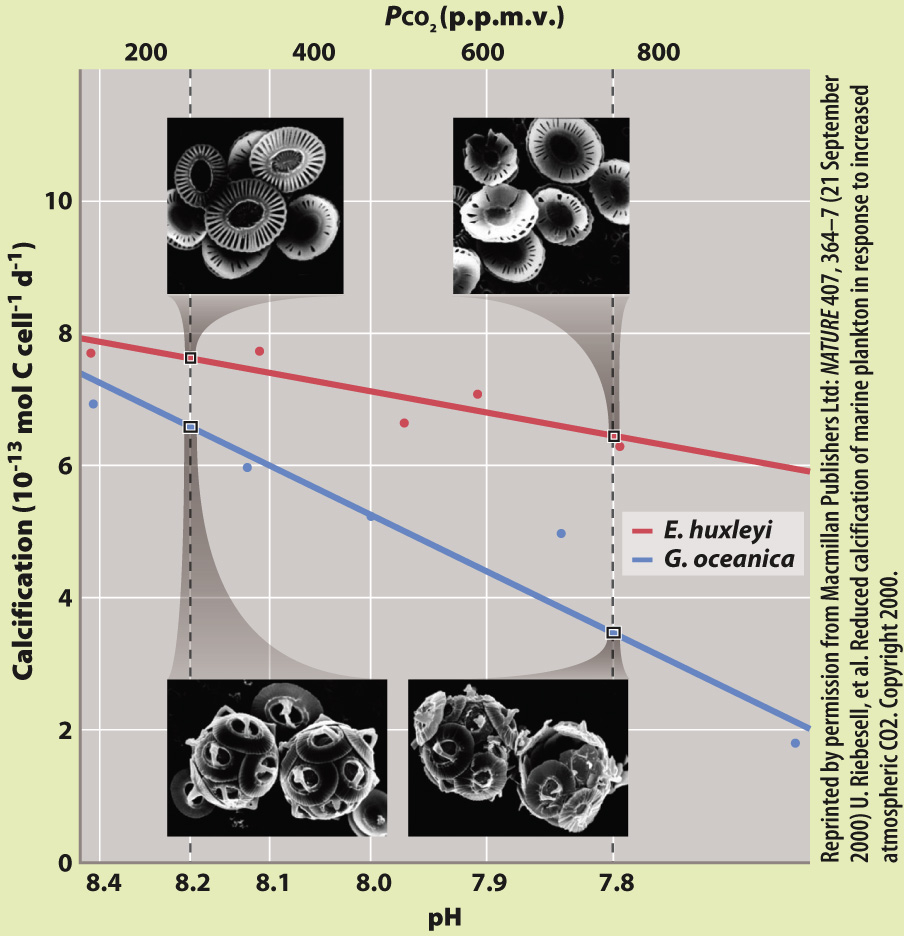
FIG. 49.14
Reprinted by permission from Macmillan Publishers Ltd: NATURE 407, 364–7 (21 September 2000) U. Riebesell, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2. Copyright 2000.
RESULTS With increasing CO2 and decreasing pH, they observed decreasing rates of calcification as well as deformed and incomplete coccolithophorids under the scanning electron microscope.
CONCLUSION The results support Riebesell’s hypothesis: Ocean acidification interferes with normal skeleton formation in marine plankton.
FOLLOW-UP WORK This work has been extended to investigate the effects of ocean acidification on skeleton formation in corals. For example, the Mediterranean coral Oculina patagonica grows normally at pH 8.2. When grown at pH 7.4, however, the coral skeletons dissolve. Upon restoration of normal conditions, the corals resume skeleton formation. This experiment shows that corals might survive ocean acidification, although the ecosystem benefits provided by reefs would be lost.
SOURCES Riebesell, U., et al. 2000. “Reduced Calcification of Marine Plankton in Response to Increased Atmospheric CO2.” Nature 407: 364–367; Fine, M., and D. Tchernov. 2007. “Scleractinian Coral Species Survive and Recover from Decalcification.” Science 315:1811–1811.
Essentially, all attempts to model our environmental future predict that, by the end of this century, atmospheric CO2 will rise from its current level to more than 500 ppm, and changing pH in many parts of the ocean will limit the ability of corals to build skeletons. Some corals can survive this change. For example, experiments on two Mediterranean corals showed that at a pH level 0.8 units below that of present-day seawater, the corals grew naked—essentially as sea anemones—and recovered their skeletons upon return to normal seawater. But, if ocean acidification persists over timescales much longer than coral generations, the structural framework for tropical reefs may be irrevocably lost.



