Most proteins are composed of modular folding domains.
If functional proteins are so unlikely, how could life have evolved? The answer is that the earliest proteins were probably much shorter than modern proteins and needed only a trace of function. Only as proteins evolved through billions of years did they become progressively longer and more specialized in their functions. Many protein families that exist today exhibit small regions of three-
Two examples of folding domains are illustrated in Fig. 4.20. Many folding domains are functional units in themselves. The folding domain called a TIM barrel (Fig. 4.20a) is named after the enzyme triose phosphate isomerase, in which it is a prominent feature. The TIM barrel consists of alternating α helices and parallel β sheets connected by loops. In many enzymes with a TIM barrel, the active site is formed by the loops at the carboxyl ends of the sheets. Fig. 4.20b is a β barrel formed from antiparallel β sheets. β barrel structures occur in proteins in some types of bacteria, usually in proteins that span the cell membrane, where the β barrel provides a channel that binds hydrophilic molecules.
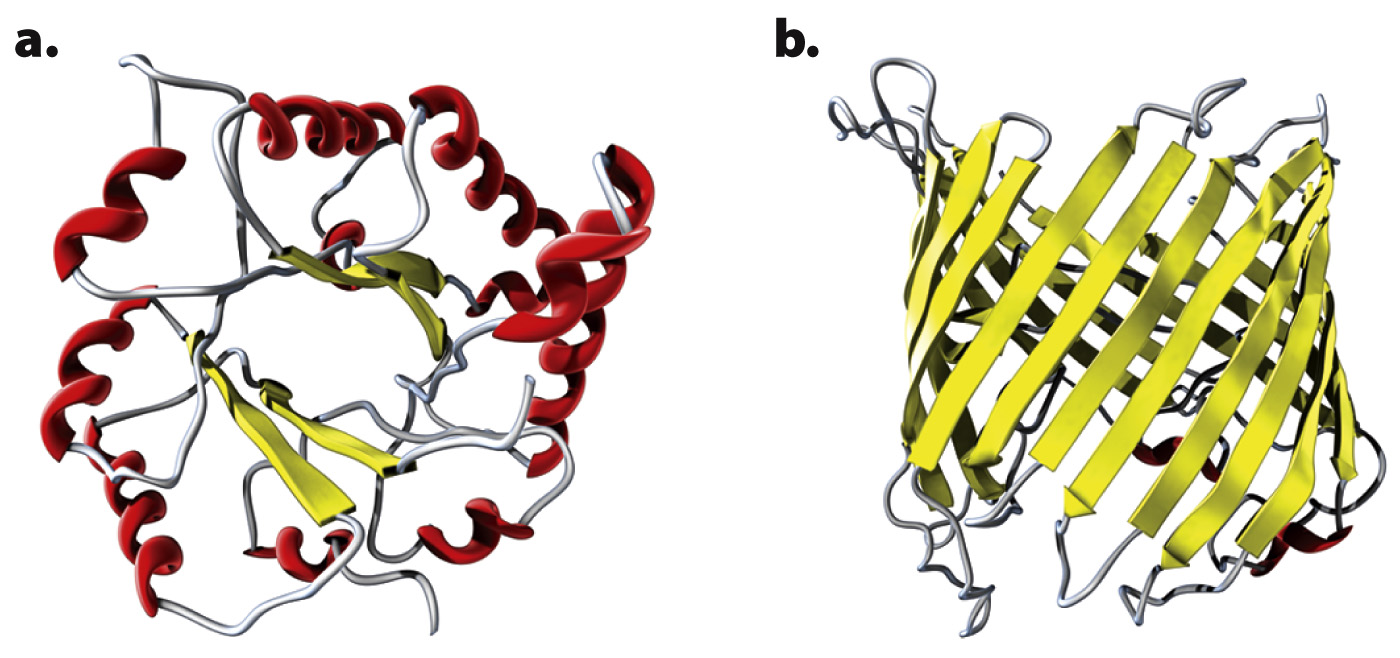
The number of known folding domains is only about 2500, which is far fewer than the number of protein families. The reason for the discrepancy is that different protein families contain different combinations of folding domains. Modern protein families are composed of different combinations of a number of folding domains, each of which contributes some structural or functional feature of the protein. Different types of protein folds occur again and again in different contexts and combinations. The earliest proteins may have been little more than single folding domains that could aggregate to form more complex functional units. As life evolved, the proteins became longer by joining the DNA coding for the individual folding units together into a single molecule.
For example, human tissue plasminogen activator, a protein that is used in treating strokes and heart attacks because it dissolves blood clots, contains domains shared with cell-