Protein sorting directs proteins to their proper location in or out of the cell.
As we have seen, eukaryotic cells have many compartments, and different proteins function in different places, such as enzymes in lysosomes or transmembrane proteins embedded in the plasma membrane. Protein sorting is the process by which proteins end up where they need to be to perform their function. Protein sorting directs proteins to the cytosol, the lumen of organelles, the membranes of the endomembrane system, or even out of the cell entirely.
Recall that proteins are produced in two places: free ribosomes in the cytosol and membrane-bound ribosomes on the rough ER. Proteins produced on free ribosomes are sorted after they are translated. These proteins often contain amino acid sequences, called signal sequences, that allow them to be recognized and sorted. As shown in Fig. 5.23, there are several types of signal sequences that direct proteins synthesized on free ribosomes to different cellular compartments. Proteins with no signal sequence remain in the cytosol (Fig. 5.23a). Proteins destined for mitochondria or chloroplasts often have a signal sequence at their amino ends (Fig. 5.23b). Proteins targeted to the nucleus usually have signal sequences located internally (Fig. 5.23c). These nuclear signal sequences, called nuclear localization signals, enable proteins to move through pores in the nuclear envelope
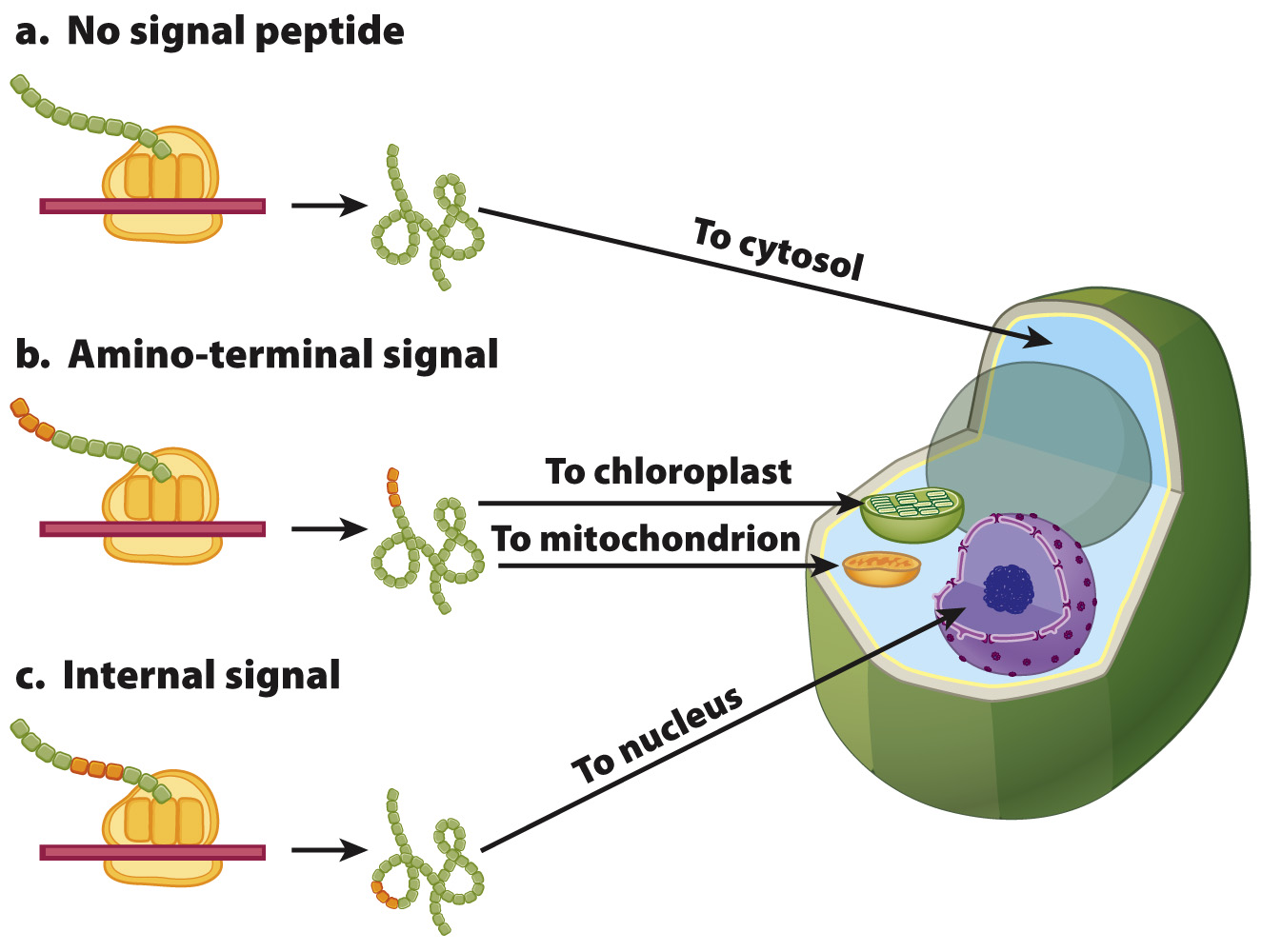
FIG. 5.23 Signal sequences on proteins synthesized by free ribosomes. (a) Most proteins with no signal peptide remain in the cytosol. Other signal sequences direct proteins to (b) mitochondria and chloroplasts or to (c) the nucleus.
Page 109
Proteins produced on the rough ER, as we have seen, end up in the lumen of the endomembrane system, secreted out of the cell, or as transmembrane proteins (Fig. 5.24). These proteins are sorted as they are translated. They begin translation on free ribosomes, but a specific signal sequence at their amino terminal end directs the ribosome to the rough ER and into a membrane channel that leads into the ER lumen. If the polypeptide contains no other signal sequence, it continues into the lumen. If it contains a second sequence, called a signal-anchor sequence, it does not continue all the way into the lumen and ends up in the membrane. Let’s consider in more detail how sorting of these proteins happens.
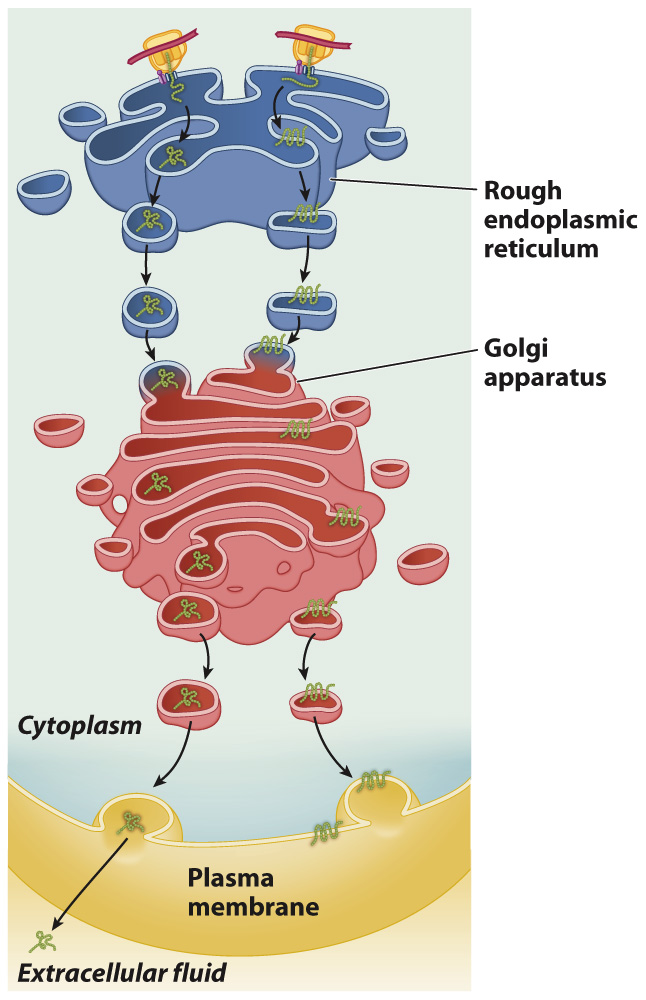
FIG. 5.24 Pathways for proteins destined (left) to be secreted or (right) transported to the plasma membrane.
Proteins destined for the ER lumen or secretion have an amino-terminal signal sequence, shown in Fig. 5.25. As a free ribosome translates the protein, this sequence is recognized by an RNA–protein complex known as a signal-recognition particle (SRP). The SRP binds to both the signal sequence and the free ribosome, and brings about a pause in translation (Fig. 5.25a). The SRP then binds with a receptor on the RER so that the ribosome is now associated with the RER (Fig. 5.25b). The SRP receptor brings the ribosome to a channel in the membrane of the RER. The SRP then dissociates and translation continues, allowing the growing polypeptide chain to be threaded through the channel (Fig. 5.25c). A specific protease cleaves the signal sequence as it emerges in the lumen of the ER (Fig. 5.25d). Some proteins are retained in the interior of the ER and others are transported in vesicles to the interior of the Golgi apparatus. Some of these proteins are secreted by exocytosis.
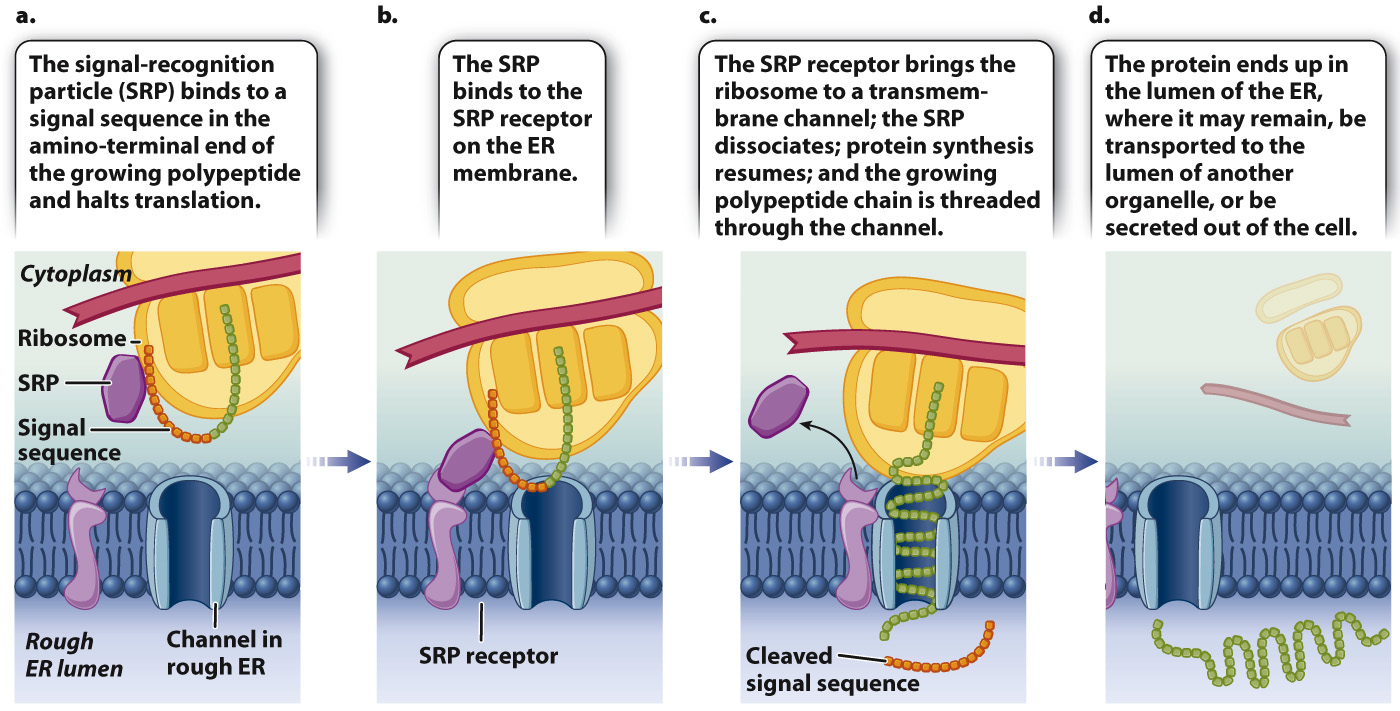
FIG. 5.25 Interaction of a signal sequence, signal-recognition particle (SRP), and SRP receptor. Binding of a signal sequence with a signal-recognition particle (SRP) halts translation, followed by docking of the ribosome on the ER membrane, release of the SRP, and continuation of translation.
Page 110
Proteins destined for cell membranes contain a signal-anchor sequence in addition to the amino-terminal signal sequence (Fig. 5.26). After the growing polypeptide chain and its ribosome are brought to the ER, it is threaded through the channel in the ER membrane until the signal-anchor sequence is encountered (Fig. 5.26a). The signal-anchor sequence is hydrophobic and is therefore able to diffuse laterally in the lipid bilayer (Fig. 5.26b). At this point, the ribosome dissociates from the channel while translation continues. When translation is completed, the carboxyl end of the chain remains on the cytosolic side of the ER membrane, the amino end is in the ER lumen, and the region between them resides in the membrane (Fig. 5.26c). Transmembrane proteins such as these may stay in the membrane of the ER or end up in other internal membranes or the plasma membrane, where they serve as transporters, pumps, receptors, or enzymes.
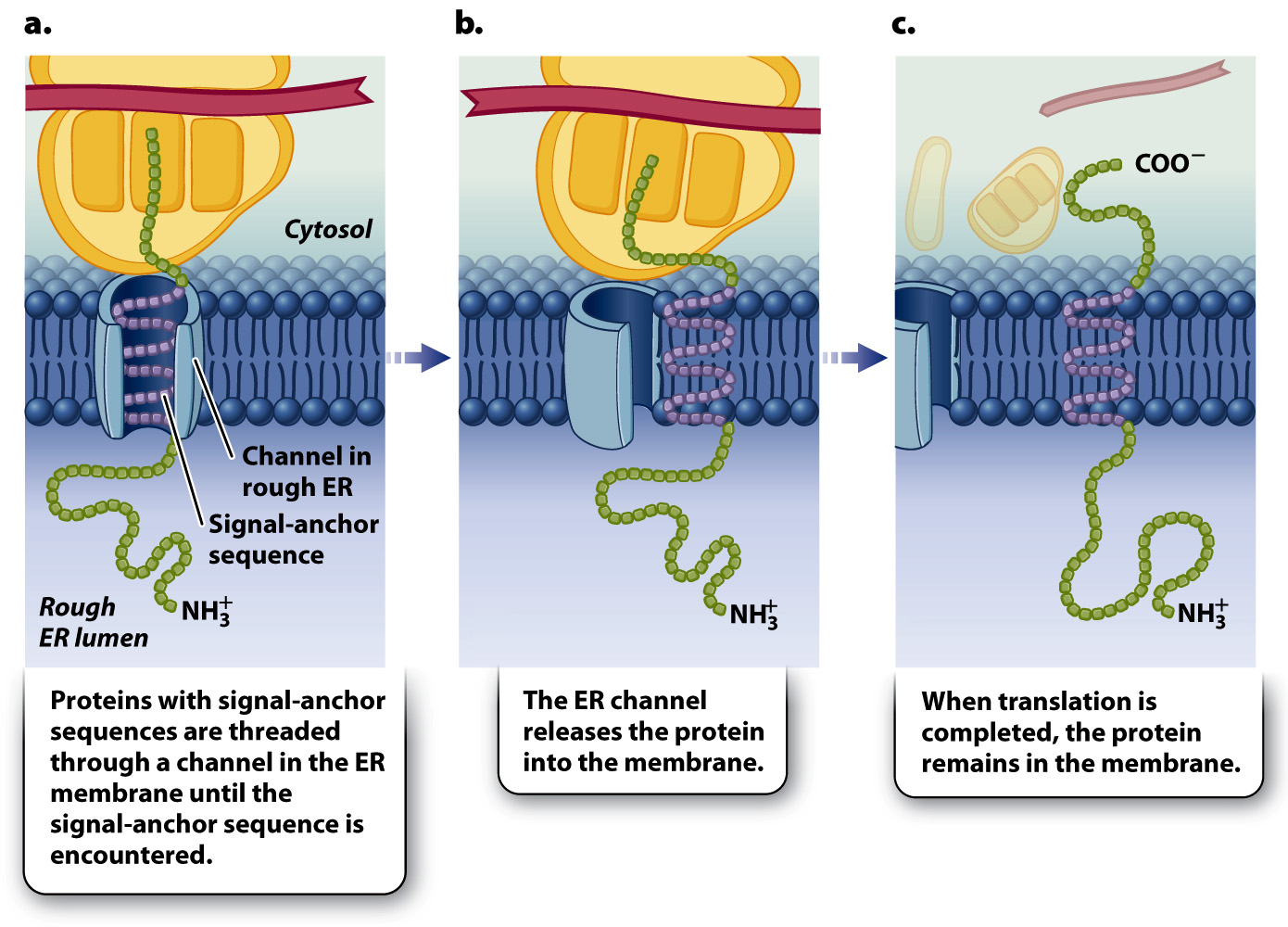
FIG. 5.26 Targeting of a transmembrane protein by means of a hydrophobic signal-anchor sequence. Proteins with a signal-anchor sequence end up embedded in the membrane.
Page 111



