The laws of thermodynamics determine whether a chemical reaction requires or releases energy available to do work.
We have seen that cells use chemical reactions to perform much of the work of the cell. The amount of energy available to do work is called Gibbs free energy (G). In a chemical reaction, we can compare the free energy of the reactants and products to determine whether the reaction releases energy that is available to do work. The difference between two values is denoted by the Greek letter delta (Δ). In this case, ΔG is the free energy of the products minus the free energy of the reactants (Fig. 6.8). If the products of a reaction have more free energy than the reactants, then ΔG is positive and a net input of energy is required to drive the reaction forward (Fig. 6.8a). By contrast, if the products of a reaction have less free energy than the reactants, ΔG is negative and energy is released and available to do work (Fig. 6.8b).
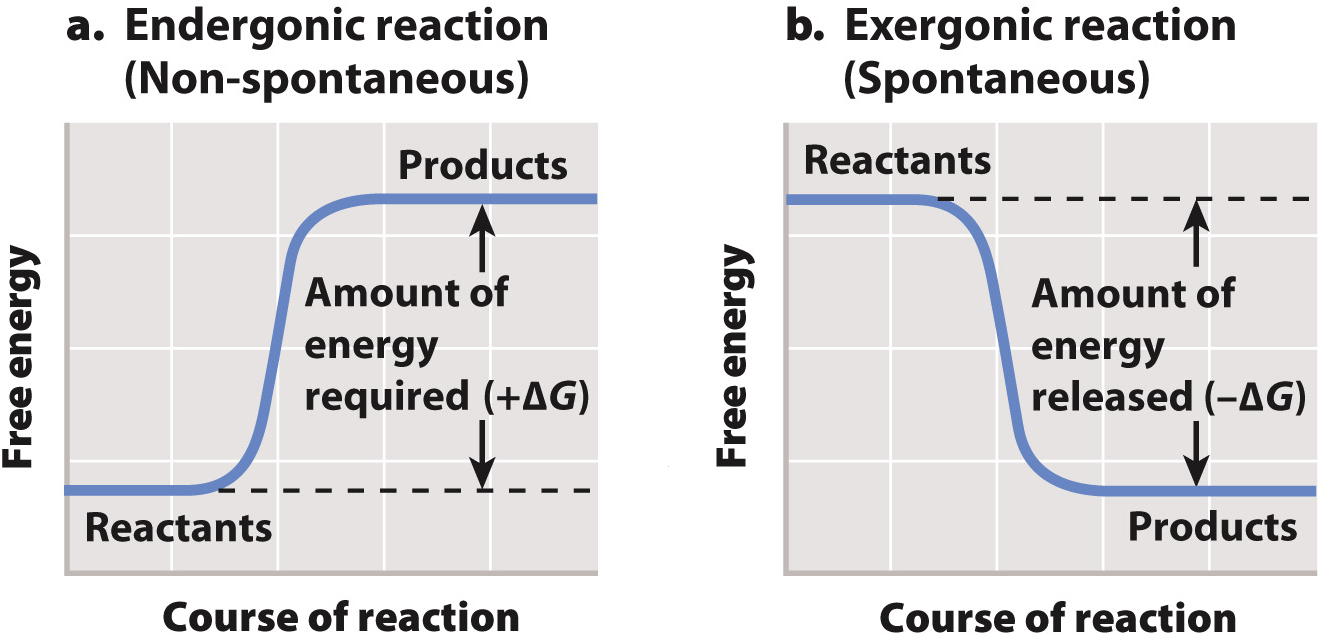
Reactions with a negative ΔG that release energy and proceed spontaneously are called exergonic, and reactions with a positive ΔG that require an input of energy and are not spontaneous are called endergonic. Note that the term “spontaneous” does not imply instantaneous or even rapid. “Spontaneous” in this context means that a reaction releases energy; “non-
Recall that the total amount of energy is equal to the energy available to do work plus the energy that is not available to do work because of the increase in entropy. We can write this relationship as an equation in which the total amount of energy is enthalpy (H), the energy available to do work is Gibbs free energy (G), and the degree of disorder is entropy (S) multiplied by the absolute temperature (T) (measured in degrees Kelvin), since temperature influences the movement of molecules (and hence the degree of disorder). Therefore,
Total amount of energy (H) = energy available to do work (G) + energy lost to entropy (TS)
We are interested in the energy available for a cell to do work, or G. Therefore, we express G in terms of the other two parameters, H and TS, as follows:
G = H – TS
In a chemical reaction, we can compare the total energy and entropy of the reactants with the total energy and entropy of the products to see if there is energy available to do work. As a result, we get
ΔG = ΔH – TΔS
This equation is a useful way to see if a chemical reaction takes place spontaneously, what direction the reaction proceeds in, and whether net energy is required or released. Let’s take a step back and make sure it makes sense intuitively. The value of ΔG depends on both the change in enthalpy and the change in disorder. Catabolic reactions are those in which the products have less chemical energy (lower enthalpy) in their bonds than the reactants have, and the products are more disordered (higher entropy) than the reactants are. In other words, such reactions have a negative value of ΔH and a positive value of ΔS. Notice that, in the equation ΔG = ΔH – TΔS, there is a negative sign in front of ΔS, so both the negative ΔH and positive ΔS contribute to a negative ΔG. Therefore, these reactions proceed spontaneously (Fig. 6.9).
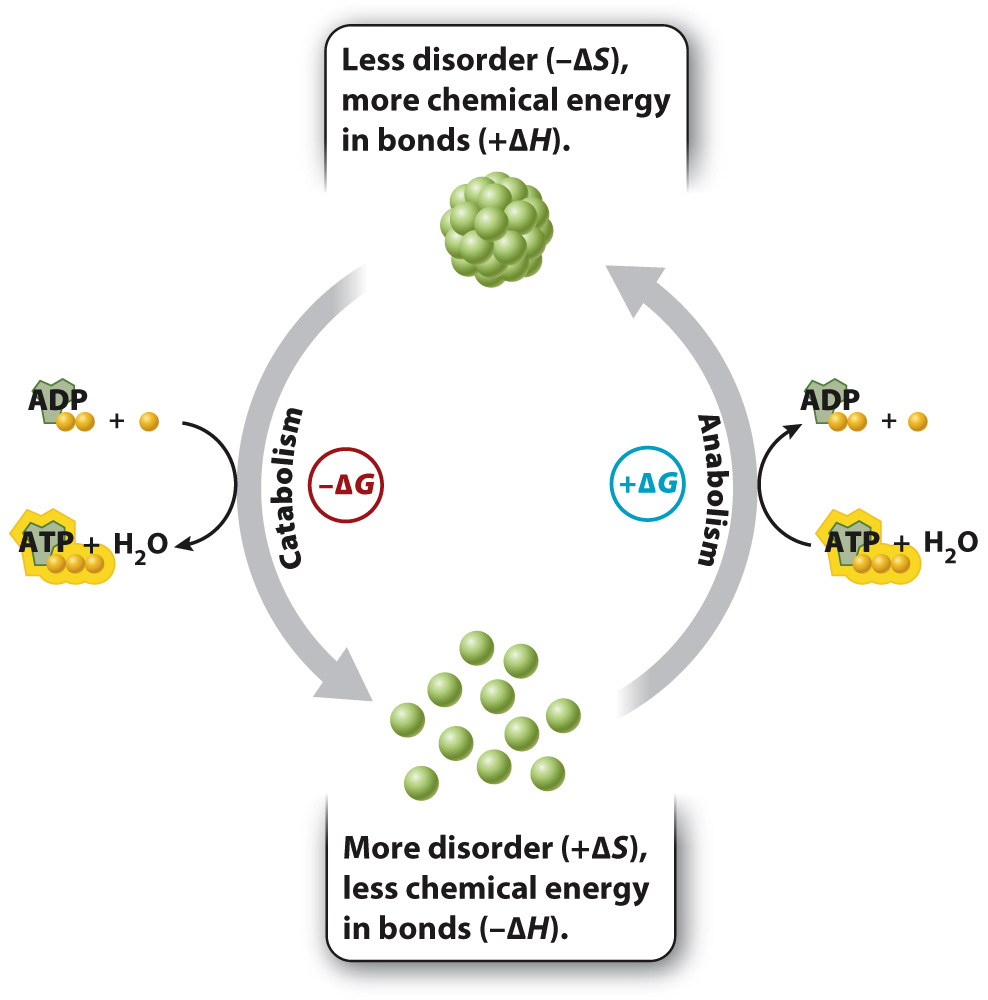
The reverse is also true: Increasing chemical energy (positive ΔH) and decreasing disorder (negative ΔS), as in the synthesis of proteins from individual amino acids and other anabolic reactions, results in a positive value of ΔG and requires a net input of energy (Fig. 6.9).
There are also cases in which the change in enthalpy and the change in entropy are both positive or both negative. In these cases, the absolute value of these parameters determines whether ΔG is positive or negative and therefore whether a reaction is spontaneous or not.
Quick Check 2 How does increasing the temperature affect the change in free energy (ΔG) of a chemical reaction?
Quick Check 2 Answer
Increasing the temperature increases the value of TΔS, which decreases ΔG, since ΔG = ΔH – TΔS. As a result, an increase in temperature makes it more likely that a reaction will proceed without a net input of energy.