Enzymes reduce the activation energy of a chemical reaction.
Earlier, we saw that exergonic reactions release free energy and endergonic reactions require free energy. Nevertheless, all chemical reactions require an input of energy to proceed, even exergonic reactions that release energy. For an exergonic reaction, the energy released is more than the initial input of energy, so there is a net release of energy.
Why is an input of energy needed for all chemical reactions? As a chemical reaction proceeds, existing chemical bonds break and new ones form. For an extremely brief period of time, a compound is formed in which the old bonds are breaking and the new ones are forming. This intermediate stage between reactants and products is called the transition state. It is highly unstable and therefore has a large amount of free energy.
In all chemical reactions, reactants adopt at least one transition state before their conversion into products. The graph in Fig. 6.13 represents the free energy levels of the reactant, transition state, and product. This reaction is spontaneous since the free energy of the reactant is higher than the free energy of the product and ΔG is negative. However, the highest free energy value corresponds to the transition state.
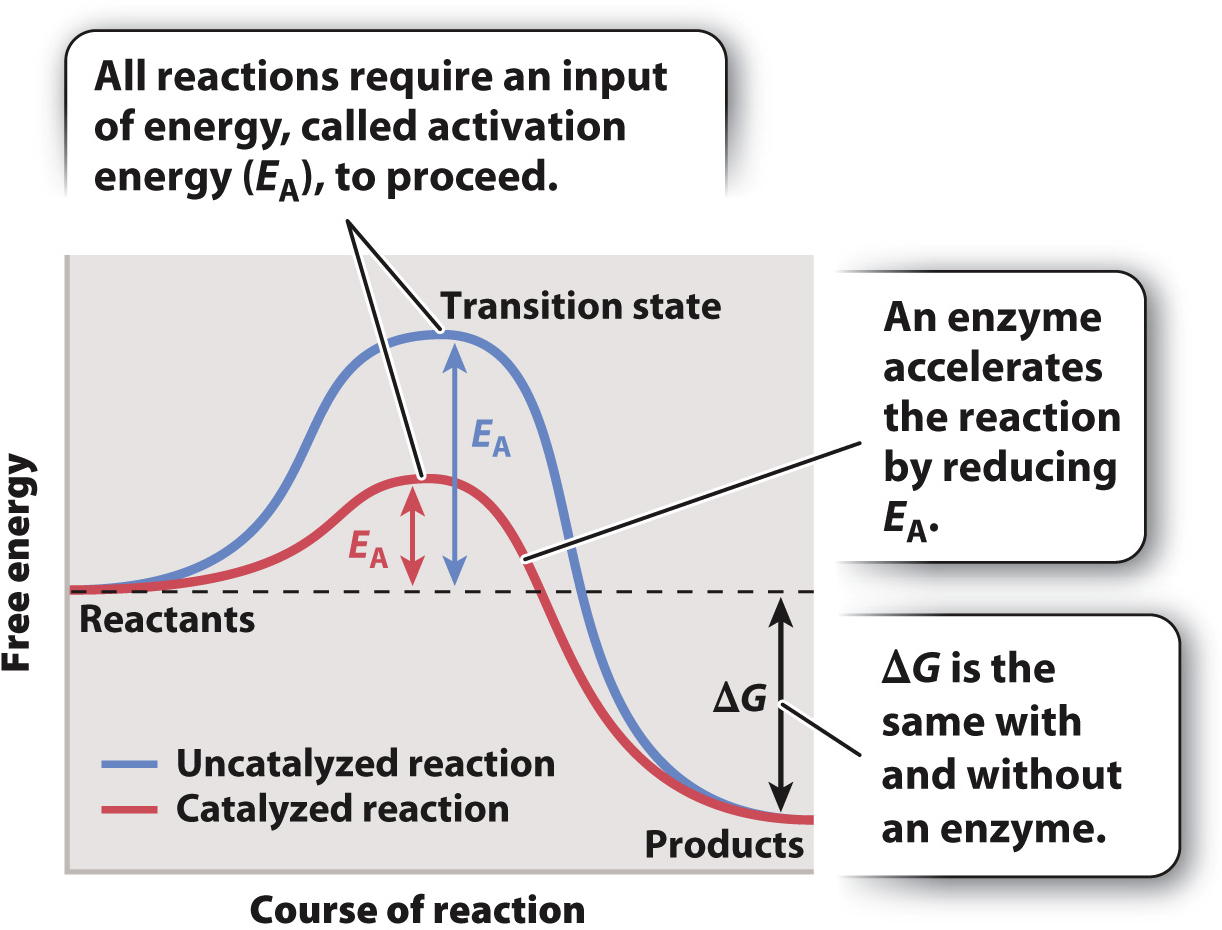
To reach the transition state, the reactant must absorb energy from its surroundings (the uphill portion of the curve in Fig. 6.13). As a result, all chemical reactions, even spontaneous ones that release energy, require an input of energy that we can think of as an “energy barrier.” The energy input necessary to reach the transition state is called the activation energy (EA). Once the transition state is reached, the reaction proceeds, products are formed, and energy is released into the surroundings (the downhill portion of the curve in Fig. 6.13). There is an inverse correlation between the rate of a reaction and the height of the energy barrier: the lower the energy barrier, the faster the reaction; the higher the barrier, the slower the reaction.
In chemical reactions that take place in the laboratory, heat is a common source of energy used to overcome the energy barrier. In living organisms, reactions are accelerated by the action of enzymes. However, enzymes do not act by supplying heat. Instead, they reduce the activation energy by stabilizing the transition state and decreasing its free energy (the red curve in Fig. 6.13). As the activation energy decreases, the speed of the reaction increases.
Although an enzyme accelerates a reaction by reducing the activation energy, the difference in free energy between reactants and products (ΔG) does not change. In other words, an enzyme changes the path of the reaction between reactants and products, but not the starting or end point (Fig. 6.13). Consider the breakdown of glucose into carbon dioxide and water. The ΔG of the reaction is the same whether it proceeds by combustion or by the action of multiple enzymes in a metabolic pathway in a cell.
Quick Check 3 Which of the following do enzymes change? ΔG; reaction rate; types of product generated; activation energy; the laws of thermodynamics.
Quick Check 3 Answer
Enzymes increase the reaction rate and decrease the activation energy. The other parameters are not changed by enzymes.