Chapter 6 Summary
Core Concepts Summary
6.1 Metabolism is the set of biochemical reactions that transforms biomolecules and transfers energy.
Organisms can be grouped according to their source of energy: Phototrophs obtain energy from sunlight and chemotrophs obtain energy from chemical compounds. page 116
Organisms can also be grouped according to the source of carbon they use to build organic molecules: Heterotrophs obtain carbon from organic molecules, and autotrophs obtain carbon from inorganic sources, such as carbon dioxide. page 116
Catabolism is the set of reactions that break down molecules and release energy, and anabolism is the set of reactions that build molecules and require energy. page 117
6.2 Kinetic energy is energy of motion and potential energy is stored energy.
Kinetic energy is due to motion. page 118
Potential energy depends on the structure of an object or its position relative to its surroundings. page 118
Chemical energy is a form of potential energy held in the bonds of molecules. page 118
6.3 The laws of thermodynamics govern energy flow in biological systems.
The first law of thermodynamics states that energy cannot be created or destroyed. page 119
The second law of thermodynamics states that there is an increase in entropy in the universe over time. page 120
6.4 Chemical reactions involve the breaking and forming of bonds.
In a chemical reaction, atoms themselves do not change, but which atoms are linked to each other changes, forming new molecules. page 121
The direction of a chemical reaction is influenced by the concentration of reactants and products. page 121
Gibbs free energy (G) is the amount of energy available to do work. page 121
Three thermodynamic parameters define a chemical reaction: Gibbs free energy (G), enthalpy (H), and entropy (S). page 121
Exergonic reactions are spontaneous (ΔG < 0) and release energy. page 121
Endergonic reactions are non-
The change of free energy in a chemical reaction is described by ΔG = ΔH – TΔS. page 122
The hydrolysis of ATP is an exergonic reaction that drives many endergonic reactions in a cell. page 122
In living systems, non-
6.5 The rate of biochemical reactions is increased by protein catalysts called enzymes.
Enzymes reduce the free energy level of the transition state between reactants and products, thereby reducing the energy input, or activation energy, required for a chemical reaction to proceed. page 124
During catalysis, the substrate and product form a complex with the enzyme. Transient covalent bonds and/or weak noncovalent interactions stabilize the complex. page 125
The size of the active site of an enzyme is small compared to the size of the enzyme as a whole and the active site amino acids occupy a very specific spatial arrangement. page 125
An enzyme is highly specific for its substrate and for the types of reaction it catalyzes. page 126
Inhibitors reduce the activity of enzymes and can act irreversibly or reversibly. page 127
Activators increase the activity of enzymes. page 127
Allosteric enzymes bind activators and inhibitors at sites other than the active site, resulting in a change in their shape and activity. page 127
Allosteric enzymes are often found at or near the start of a metabolic pathway or at the crossroads of multiple pathways. page 128
Self-Assessment
Name and describe ways that organisms obtain energy and carbon from the environment.
Self-Assessment 1 Answer
Organisms obtain energy from the environment in two ways: (1) by harvesting energy from sunlight (phototrophs), and (2) by harvesting energy from chemical compounds (chemotrophs). These groups are further distinguished by how they obtain carbon. Autotrophs obtain carbon directly from inorganic sources—
such as carbon dioxide— and convert it into an organic source of carbon, like glucose. Heterotrophs obtain carbon from organic compounds made by other organisms. (See Fig. 6.1 for examples.) Distinguish between catabolism and anabolism.
Self-Assessment 2 Answer
Catabolism is the set of chemical reactions that break down macromolecules into smaller units, producing energy (ATP). Anabolism is the set of chemical reactions that build macromolecules from smaller units and require an input of energy (usually in the form of ATP).
Name and describe the two forms of energy and provide an example of each.
Self-Assessment 3 Answer
Energy is a system’s capacity to do work. One form of energy is kinetic energy (the energy of motion). Moving objects perform work that results in their movement and the movement of surrounding matter. Examples of kinetic energy are flexing a muscle, throwing a ball, and basically any kind of movement. The other form of energy is potential energy (stored energy). Potential energy depends on the structure of the object or its position relative to its surroundings, and it is released by a change in the object’s structure or position. A ball sitting on the top of the stairs has a great deal of potential energy, which is released when the ball starts to roll down the stairs (at which point, the energy is converted into kinetic energy).
Explain the relationship between strength of a covalent bond and the amount of chemical energy it contains.
Self-Assessment 4 Answer
Chemical energy in molecules is stored as potential energy of the electrons in atoms, occupying orbitals at various distances from the nucleus. The stronger the covalent bond, the less chemical energy it contains. The weaker the covalent bond, the more chemical energy it contains. Carbohydrates, lipids, and proteins have many carbon‒carbon and carbon‒hydrogen bonds. These bonds are relatively weak and are therefore rich sources of chemical energy.
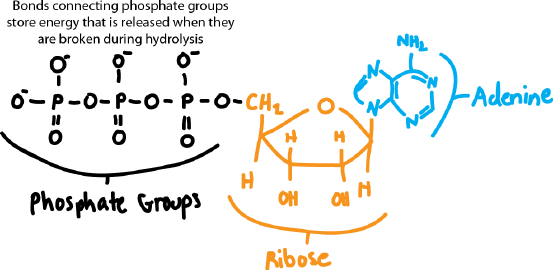
Draw the structure of ATP, indicating the bonds that are broken during hydrolysis.
Self-Assessment 5 Answer
Describe the first and second laws of thermodynamics and how they relate to chemical reactions.
Self-Assessment 6 Answer
See Figs. 6.5 and 6.6. The first law of thermodynamics is the law of conservation of energy. It states that energy can be neither created nor destroyed; it can only be transformed from one form into another. The second law of thermodynamics states that the transformation of energy is associated with an increase in the disorder of the universe. The degree of disorder is called entropy. Chemical reactions are subject to the laws of thermodynamics (like everything else). As a result, the total amount of energy remains the same before and after a chemical reaction, but some of the energy is used to increase the entropy of the system, and only some of the energy is available to do the work of the cell.
If the difference between the enthalpy of the products and that of the reactants is positive and the difference between the entropy of the products and reactants is negative, predict whether the reaction is spontaneous or not.
Self-Assessment 7 Answer
The amount of energy available to do work is called Gibbs free energy (G). In a chemical reaction, you compare the free energy of the reactants and products to determine whether there is energy available to do work. This difference is called ΔG. ΔG = ΔH ‒ TΔS. If the enthalpy difference (ΔH) is negative and the entropy difference (ΔS) is positive, then ΔG is negative and free energy is released. This kind of reaction is exergonic and occurs spontaneously.
Describe how the hydrolysis of ATP can drive non-
spontaneous reactions in a cell. Self-Assessment 8 Answer
The hydrolysis of ATP releases energy. This energy can be used to drive nonspontaneous reactions in a cell if the total ΔG for the entire pathway is negative. See Fig. 6.11.
Give three characteristics of enzymes and describe how they permit chemical reactions to occur in cells.
Self-Assessment 9 Answer
Enzymes reduce the activation energy of a chemical reaction (or the energy input necessary to reach the transition state) by stabilizing the transition state and decreasing its free energy. See Fig. 6.13. Enzymes are catalysts that participate in a chemical reaction, forming complexes with products and reactants, but are not themselves consumed in the process. See Fig. 6.14. Enzymes are also highly specific. They typically catalyze only one reaction, recognizing a specific substrate. Finally, inhibitors and activators can influence enzyme activity. Inhibitors decrease the activity of enzymes, whereas activators increase this activity. See Fig. 6.17 for an example of two different types of inhibitors.
Explain how protein folding allows for enzyme specificity.
Self-Assessment 10 Answer
An enzyme’s three-
dimensional shape is very important for its specificity. The enzyme has to fold into its correct shape in order for the active site (the portion of the enzyme that binds substrate and converts it to product) to be in the right area and shape. An enzyme will only recognize its substrate if its active site is in the right conformation due to the correct three- dimensional folding.