ATP synthase converts the energy of the proton gradient into the energy of ATP.
In 1961, Peter Mitchell proposed a hypothesis to explain how the energy stored in the proton electrochemical gradient is used to synthesize ATP. In 1978, he was awarded the Nobel Prize in Chemistry for work that fundamentally changed the way we understand how energy is harnessed by a cell.
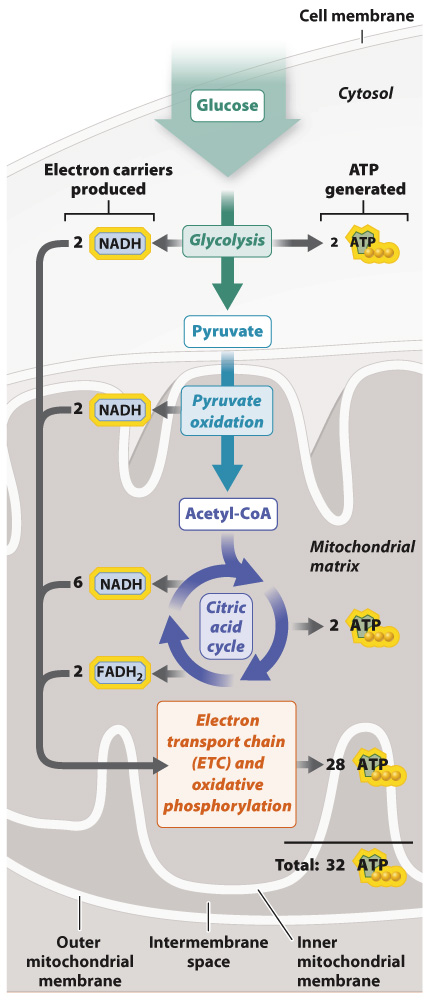
According to Mitchell’s hypothesis, the gradient of protons provides a source of potential energy that is converted into chemical energy stored in ATP. First, for the potential energy of the proton gradient to be released, there must be an opening in the membrane for the protons to flow through. Mitchell suggested that protons in the intermembrane space diffuse down their electrical and concentration gradients through a transmembrane protein channel into the mitochondrial matrix. Second, the movement of protons through the enzyme must be coupled with the synthesis of ATP. This coupling is made possible by ATP synthase, a remarkable enzyme composed of two distinct subunits called Fo and F1 (Fig. 7.11). Fo forms the channel in the inner mitochondrial membrane through which protons flow; F1 is the catalytic unit that synthesizes ATP. Proton flow through the channel (Fo) makes it possible for the enzyme (F1) to synthesize ATP.
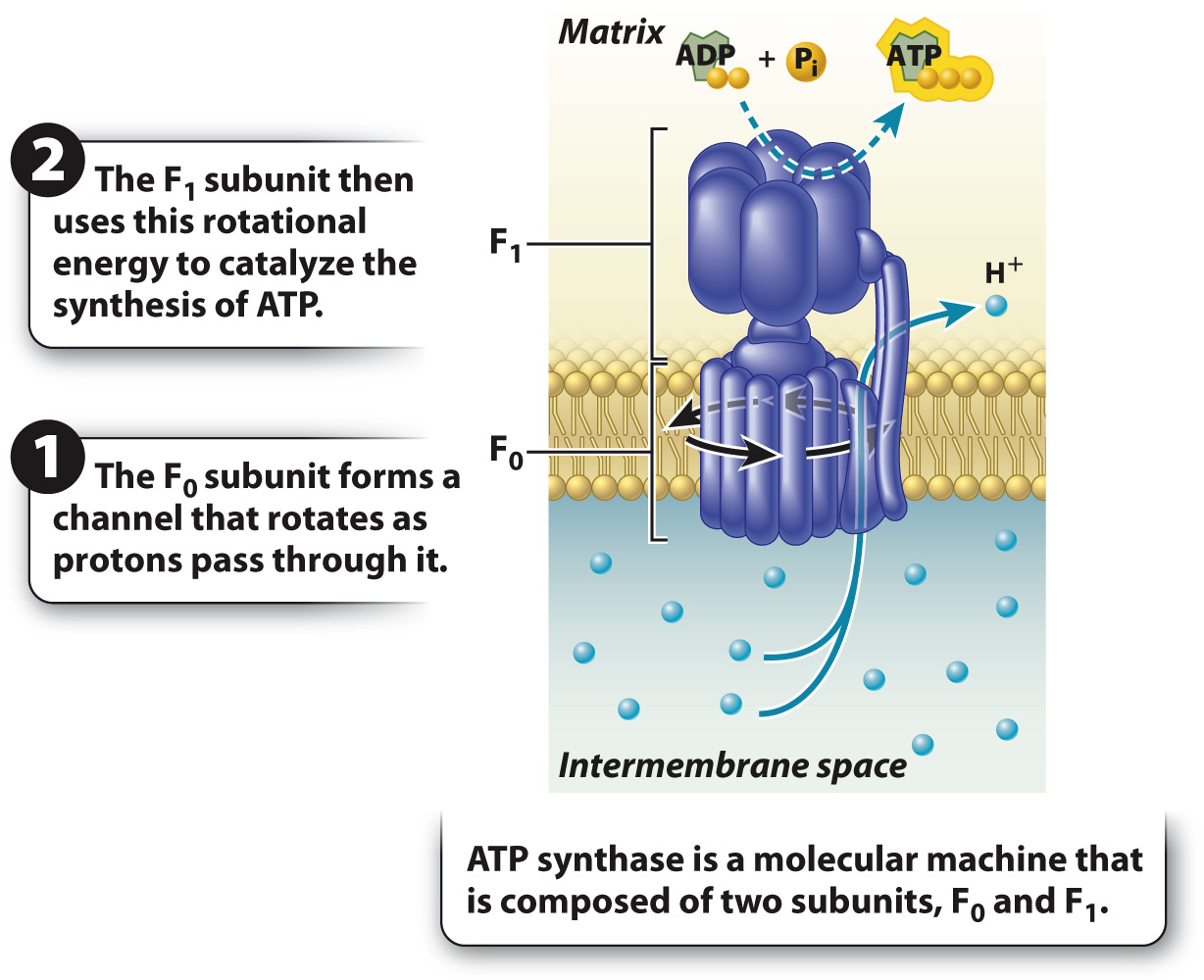
Proton flow through the Fo channel causes it to rotate, converting the energy of the proton gradient into mechanical rotational energy, a form of kinetic energy. The rotation of the Fo subunit leads to rotation of the F1 subunit in the mitochondrial matrix (Fig. 7.11). The rotation of the F1 subunit in turn causes conformational changes that allow it to catalyze the synthesis of ATP from ADP and Pi. In this way, mechanical rotational energy is converted into the chemical energy of ATP.
Direct experimental evidence for Mitchell’s idea, called the chemiosmotic hypothesis, did not come for over a decade. One of the key experiments that provided support for his idea is illustrated in Fig. 7.12.
HOW DO WE KNOW?
FIG. 7.12
Can a proton gradient drive the synthesis of ATP?
BACKGROUND Peter Mitchell’s hypothesis that a proton gradient can drive the synthesis of ATP was met with skepticism because it was proposed before experimental evidence supported it. In the 1970s, biochemist Efraim Racker and his collaborator Walther Stoeckenius tested the hypothesis.
EXPERIMENT Racker and Stoeckenius built an artificial system consisting of a membrane, a bacterial proton pump activated by light, and ATP synthase.
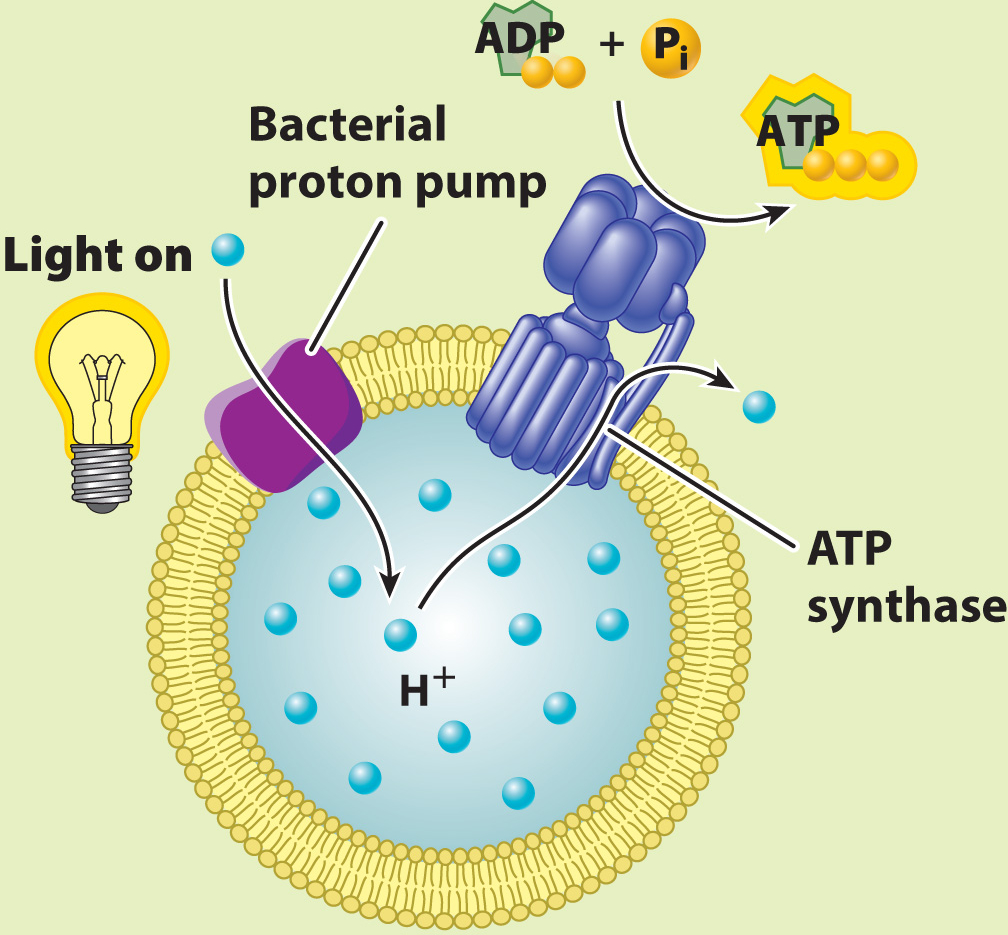
They measured the concentration of protons in the external medium and the amount of ATP produced in the presence and absence of light.
RESULTS In the presence of light, the concentration of protons increased inside the vesicles, suggesting that protons were taken up by the vesicles.
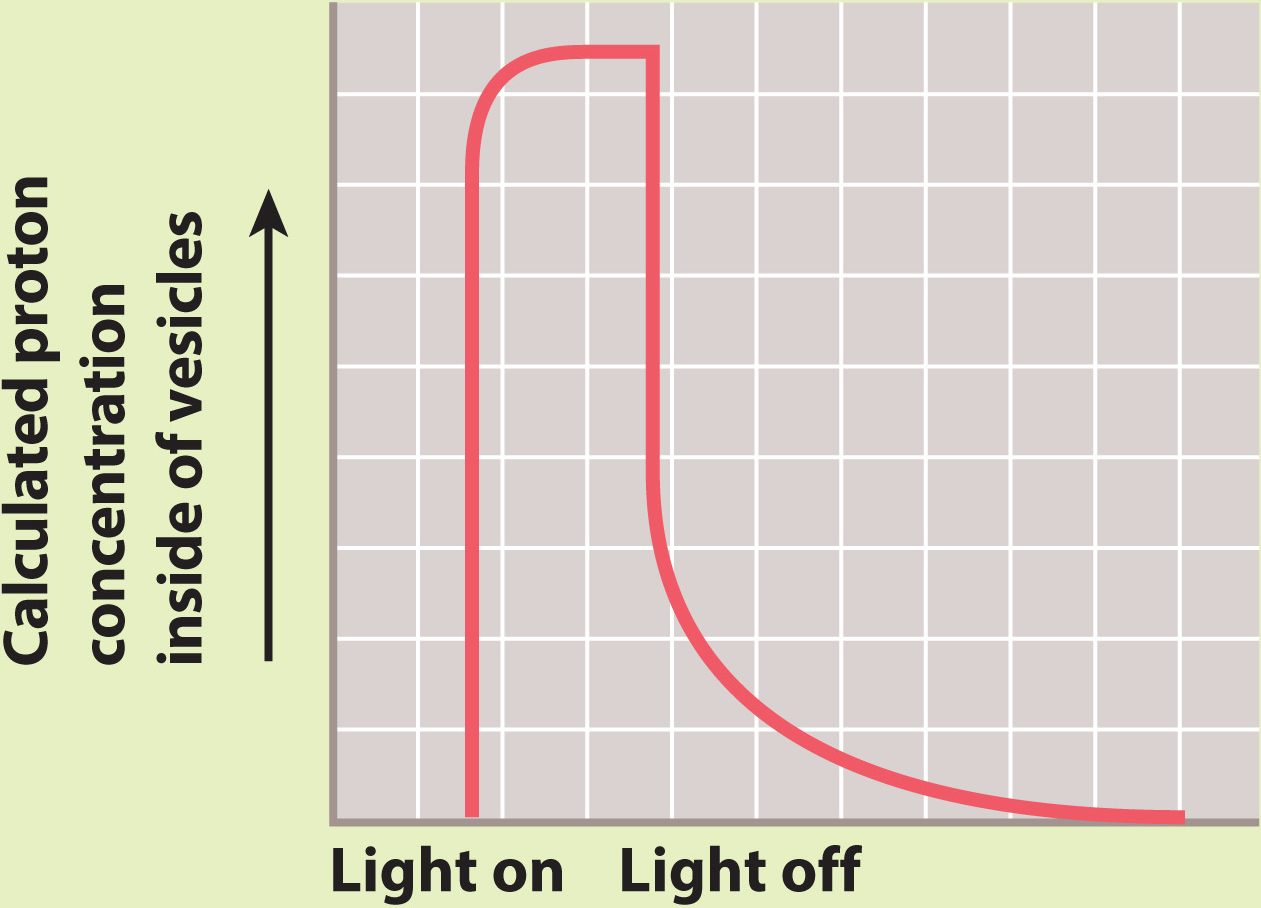
In the dark, the concentration of protons returned to the starting level. ATP was generated in the light, but not in the dark.

INTERPRETATION In the presence of light, the proton pump was activated and protons were pumped to one side of the membrane, leading to the formation of a proton gradient. The proton gradient, in turn, powered synthesis of ATP by ATP synthase.
CONCLUSION A membrane, proton gradient, and ATP synthase are sufficient to synthesize ATP. This result provided experimental evidence for Mitchell’s hypothesis.
SOURCES Mitchell, P. 1961. “Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemiosmotic Type of Mechanism.” Nature 191:144–
Quick Check 5 Uncoupling agents are proteins spanning the inner mitochondrial membrane that allow protons to pass through the membrane and bypass the channel of ATP synthase. Describe the consequences to the proton gradient and ATP production.
Quick Check 5 Answer
Uncoupling agents decrease the proton gradient and therefore decrease levels of ATP. The energy of the proton gradient is not used for oxidative phosphorylation, but instead is dissipated as heat. Uncoupling agents are found naturally in certain tissues, such as fat, for heat generation. They can also act as poisons.
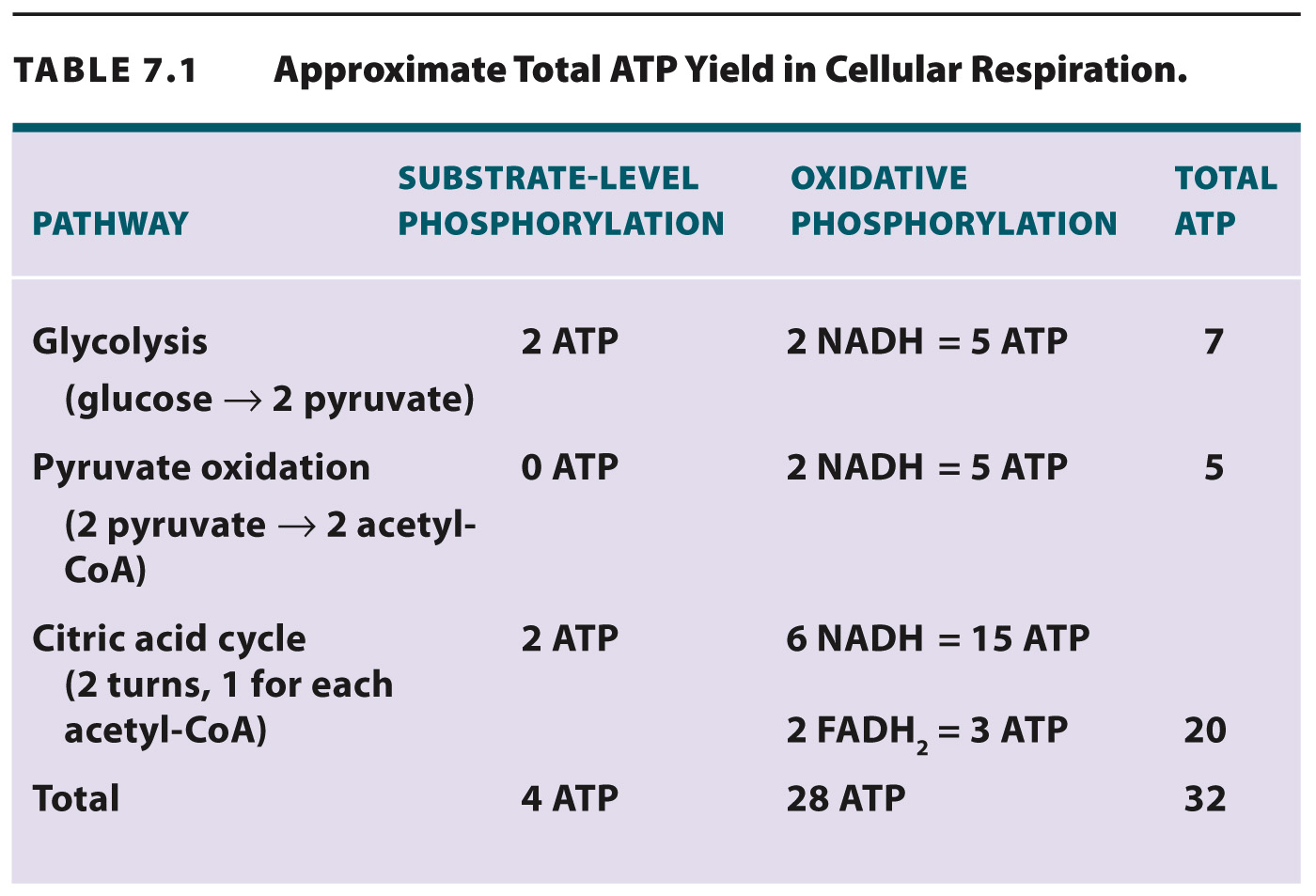
Approximately 2.5 molecules of ATP are produced for each NADH that donates electrons to the chain and 1.5 molecules of ATP for each FADH2. Therefore, overall, the complete oxidation of glucose yields about 32 molecules of ATP from glycolysis, pyruvate oxidation, the citric acid cycle, and oxidative phosphorylation (Table 7.1). Some of the energy held in the bonds of glucose is now available in a molecule that can be readily used by cells.
It is worth taking a moment to follow the flow of energy in cellular respiration, illustrated in its full form in Fig. 7.13. We began with glucose and noted that it holds chemical potential energy in its covalent bonds. This energy is released in a series of reactions and captured in chemical form. Some of these reactions generate ATP directly by substrate-