Photosystems use light energy to drive the photosynthetic electron transport chain.
When visible light is absorbed by a chlorophyll molecule, one of its electrons is elevated to a higher energy state (Fig. 8.10). For chlorophyll molecules that have been extracted from chloroplasts in the laboratory, this absorbed light energy is rapidly released, allowing the electron to return to its initial “ground” energy state (Fig. 8.10a). Most of the energy (>95%) is converted into heat; a small amount is reemitted as light (fluorescence).
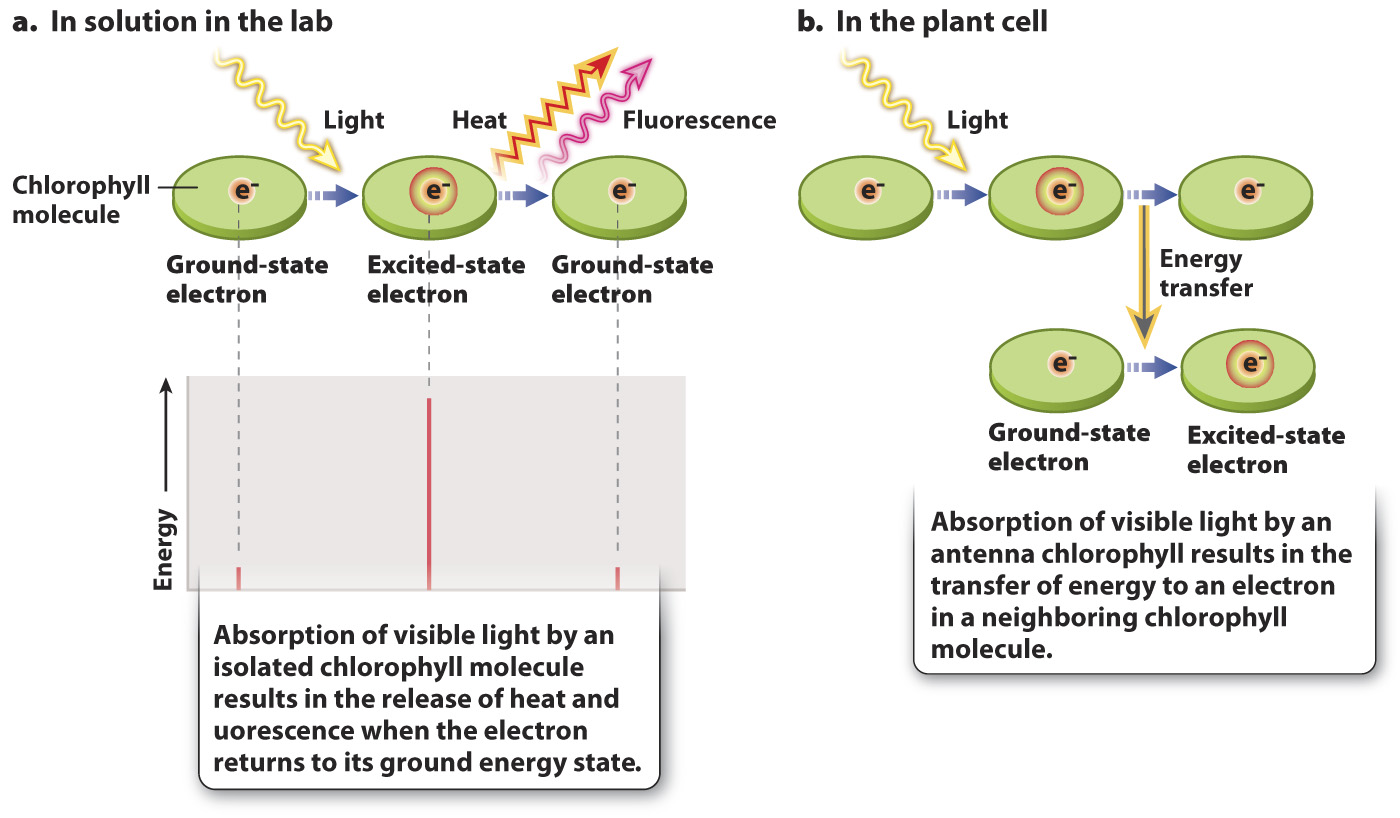
By contrast, for chlorophyll molecules within an intact chloroplast, energy can be transferred to an adjacent chlorophyll molecule instead of being lost as heat (Fig. 8.10b). When this happens, the energy released as an excited electron returns to its ground state raises the energy level of an electron in an adjacent chlorophyll molecule. This mode of energy transfer is extremely efficient (that is, very little energy is lost as heat), allowing energy initially absorbed from sunlight to be transferred from one chlorophyll molecule to another and then on to another.
Most of the chlorophyll molecules in the thylakoid membrane function as an antenna: Energy is transferred between chlorophyll molecules until it is finally transferred to a specially configured pair of chlorophyll molecules known as the reaction center (Fig. 8.11).
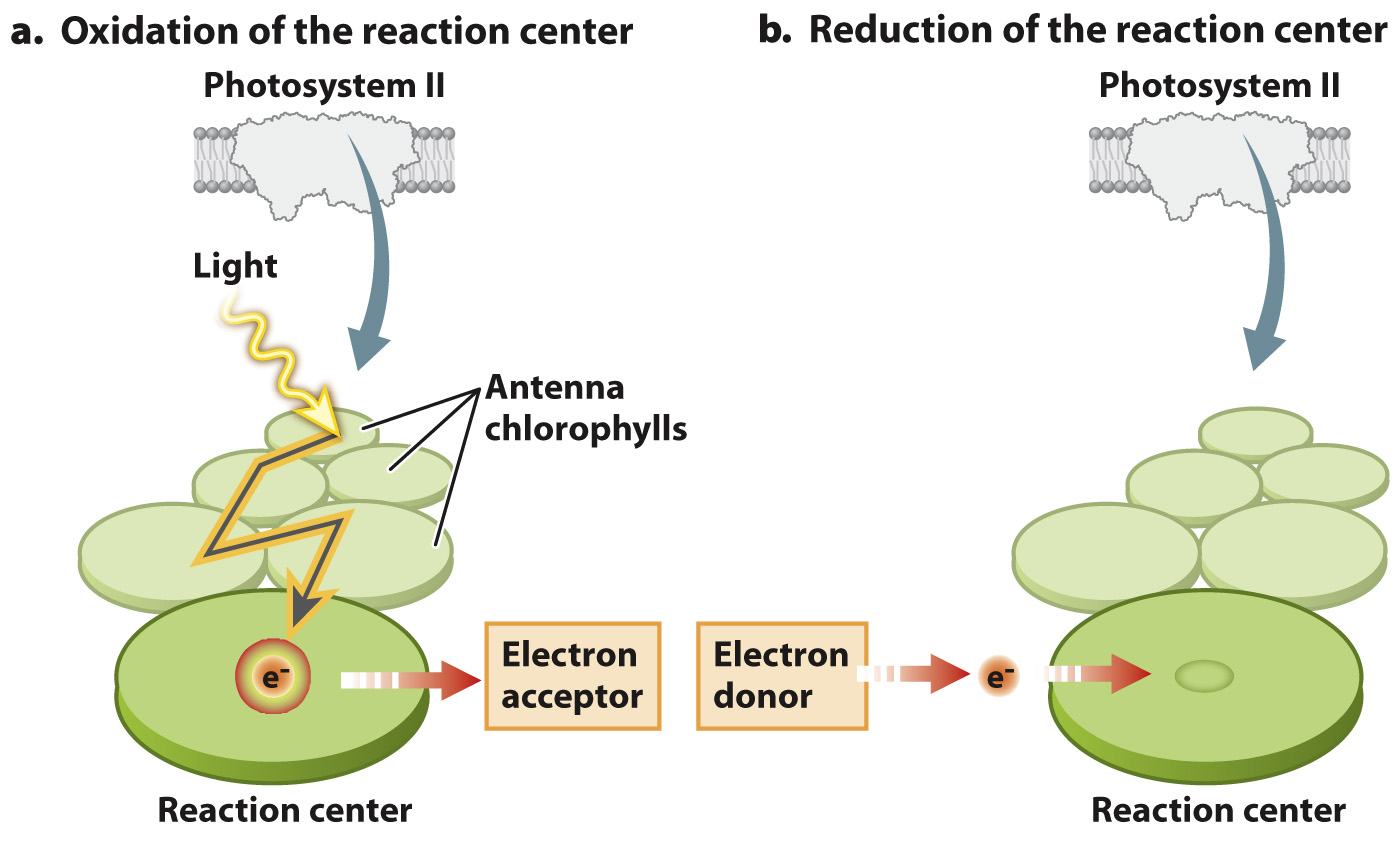
The reaction center is where light energy is converted into chemical energy as a result of the excited electron’s transfer to an adjacent molecule. This division of labor among chlorophyll molecules was discovered in the 1940s in a series of experiments by the American biophysicists Robert Emerson and William Arnold, who showed that only a small fraction of chlorophyll molecules are directly involved in electron transport (Fig. 8.12). We now know that several hundred antenna chlorophyll molecules transfer energy to each reaction center. The antenna chlorophylls allow the photosynthetic electron transport chain to operate efficiently. Without the antennae to gather light energy, reaction centers would sit idle much of the time, even in bright sunlight.
HOW DO WE KNOW?
FIG. 8.12
Do chlorophyll molecules operate on their own or in groups?
BACKGROUND By about 1915, scientists knew that chlorophyll was the pigment responsible for absorbing light energy in photosynthesis. However, it was unclear how these pigments contributed to the reduction of CO2. The American physiologists Robert Emerson and William Arnold set out to determine the nature of the “photochemical unit” by quantifying how many chlorophyll molecules were needed to produce one molecule of O2.
EXPERIMENT Emerson and Arnold exposed flasks of the green alga Chlorella to flashes of light of such short duration (10−5 s) and high intensity (several times that of full sunlight) that each chlorophyll molecule would be “excited” only once. They also made sure that the time between flashes was long enough to allow the reactions resulting from each flash to run to completion. They then measured O2 production per flash of high intensity light, and determined the concentration of chlorophyll present in their solution of cells. Finally, they divided the number of chlorophyll molecules per mm3 of solution by O2 production per flash per mm3 of cells in solution.
RESULTS
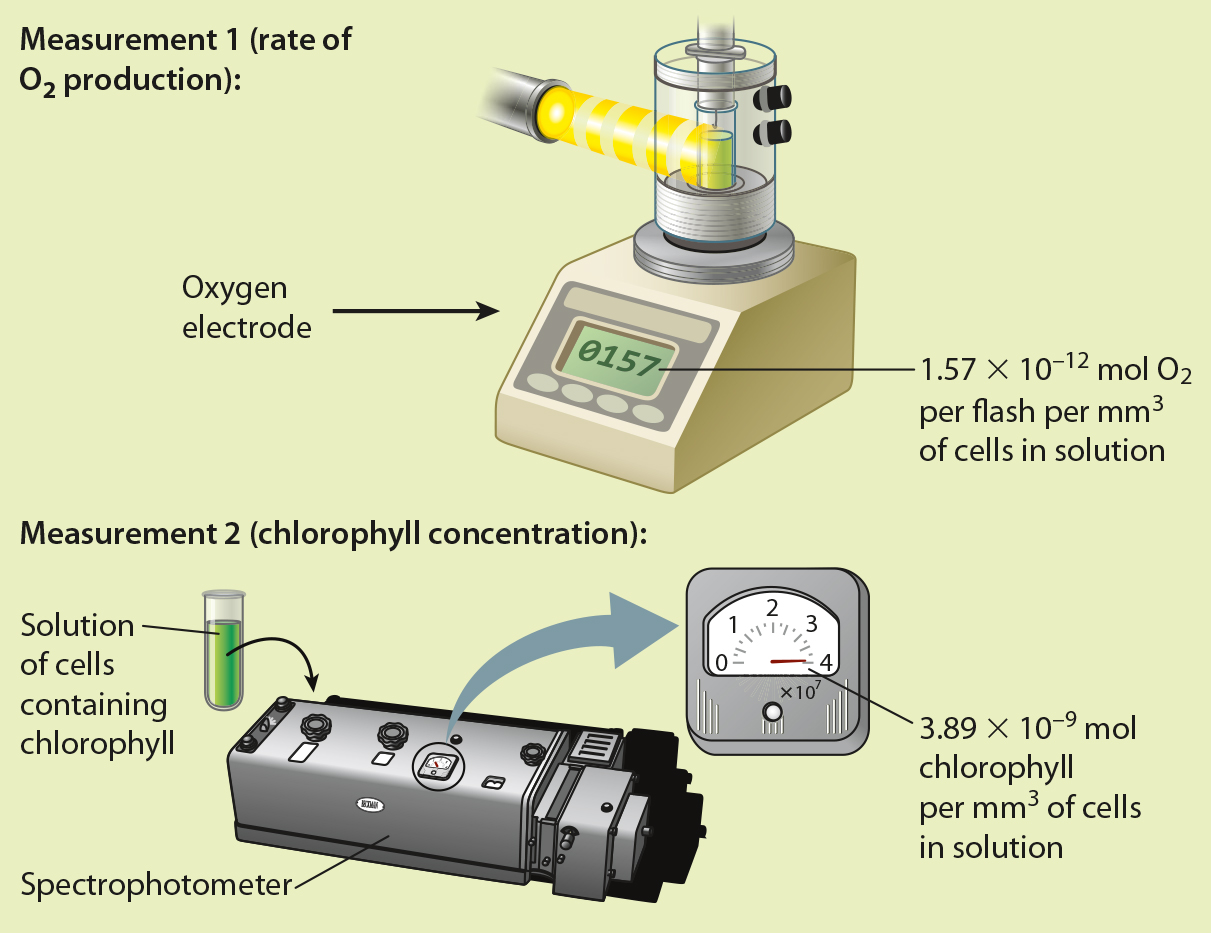
CONCLUSION Because the amount of chlorophyll in their flask was much greater than O2 production per flash, Emerson and Arnold concluded that each photochemical unit contains many chlorophyll molecules.
FOLLOW-
SOURCE Emerson, R., and W. Arnold. 1932. “The Photochemical Reaction in Photosynthesis.” Journal of General Physiology 16:191–
The reaction center chlorophylls have a configuration distinct from that of the antenna chlorophylls. As a result, when excited, the reaction center transfers an electron to an adjacent molecule that acts as an electron acceptor (Fig. 8.11a). When the transfer takes place, the reaction center becomes oxidized and the adjacent electron-
Once the reaction center has lost an electron, it can no longer absorb light or contribute additional electrons. Thus, for the photosynthetic electron transport chain to continue, another electron must be delivered to take the place of the one that has entered the transport chain (Fig. 8.11b). As we will see below, these replacement electrons ultimately come from water.
Quick Check 3 How do antenna chlorophylls differ from reaction center chlorophylls?
Quick Check 3 Answer
Antenna chlorophyll molecules transfer absorbed energy from one antenna chlorophyll molecule to another, and ultimately to the reaction center. Reaction center chlorophylls transfer electrons to an electron acceptor, resulting in the oxidation of reaction center chlorophyll molecules.