7.1 The Cerebral Cortex
7-
Older brain networks sustain basic life functions and enable memory, emotions, and basic drives. Newer neural networks within the cerebrum—the two cerebral hemispheres contributing 85 percent of the brain’s weight—
The people who first dissected and labeled the brain used the language of scholars—Latin and Greek. Their words are actually attempts at graphic description: For example, cortex means “bark,” cerebellum is “little brain,” and thalamus is “inner chamber.”
As we move up the ladder of animal life, the cerebral cortex expands, tight genetic controls relax, and the organism’s adaptability increases. Frogs and other small-
RETRIEVAL PRACTICE
- Which area of the human brain is most similar to that of less complex animals? Which part of the human brain distinguishes us most from less complex animals?
The brainstem; the cerebral cortex
Structure of the Cortex
If you opened a human skull, exposing the brain, you would see a wrinkled organ, shaped somewhat like the meat of an oversized walnut. Without these wrinkles, a flattened cerebral cortex would require triple the area—
Each hemisphere’s cortex is subdivided into four lobes, separated by prominent fissures, or folds (FIGURE 7.1). Starting at the front of your brain and moving over the top, there are the frontal lobes (behind your forehead), the parietal lobes (at the top and to the rear), and the occipital lobes (at the back of your head). Reversing direction and moving forward, just above your ears, you find the temporal lobes. Each of the four lobes carries out many functions, and many functions require the interplay of several lobes.
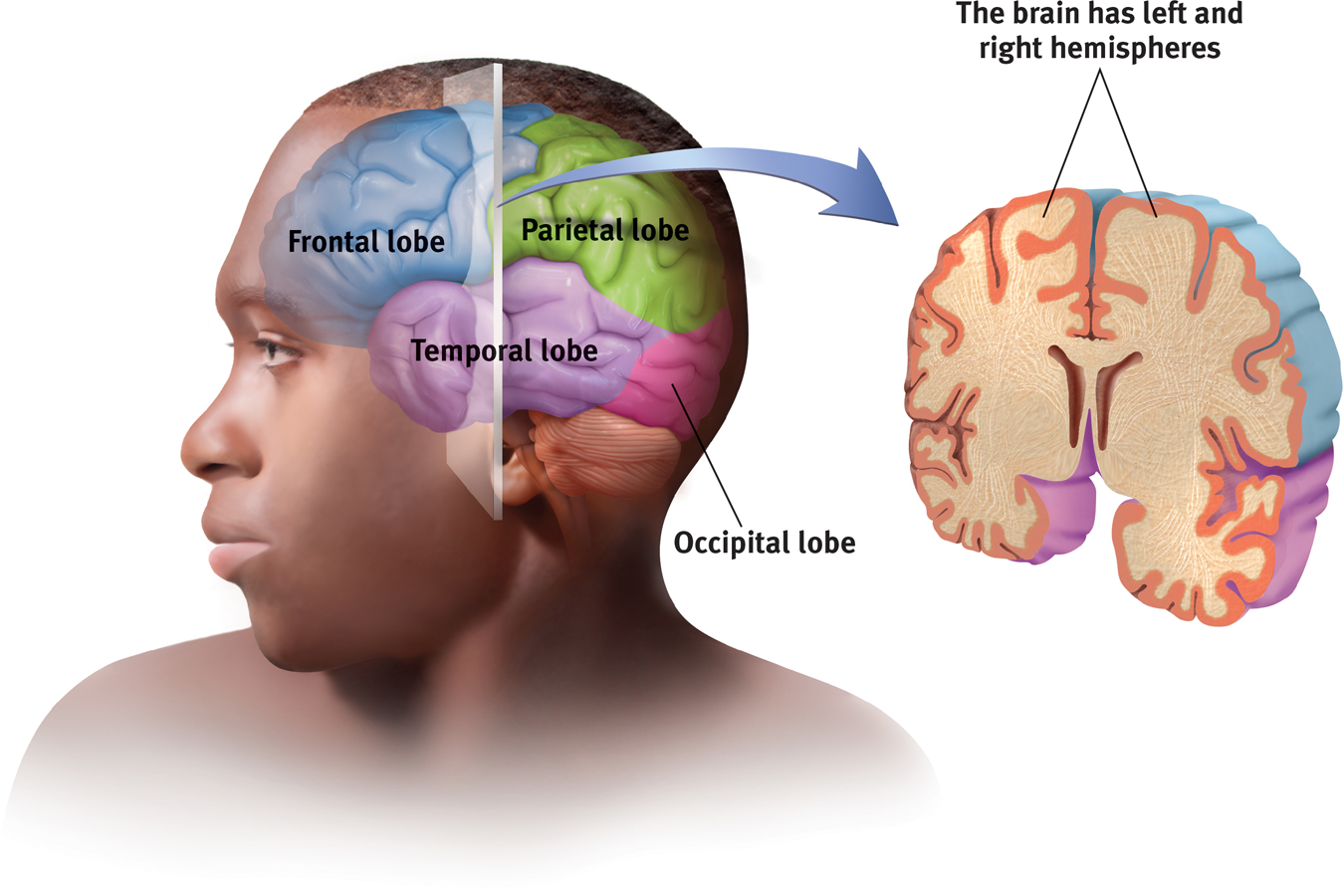
 Figure 7.1
Figure 7.1The cortex and its basic subdivisions
Functions of the Cortex
More than a century ago, surgeons found damaged cortical areas during autopsies of people who had been partially paralyzed or speechless. This rather crude evidence did not prove that specific parts of the cortex control complex functions like movement or speech. After all, if the entire cortex controlled speech and movement, damage to almost any area might produce the same effect. A TV with its power cord cut would go dead, but we would be fooling ourselves if we thought we had “localized” the picture in the cord.
Motor FunctionsScientists had better luck in localizing simpler brain functions. For example, in 1870, German physicians Gustav Fritsch and Eduard Hitzig made an important discovery: Mild electrical stimulation to parts of an animal’s cortex made parts of its body move. The effects were selective: Stimulation caused movement only when applied to an arch-
MAPPING THE MOTOR CORTEX Lucky for brain surgeons and their patients, the brain has no sensory receptors. Knowing this, Otfrid Foerster and Wilder Penfield were able to map the motor cortex in hundreds of wide-
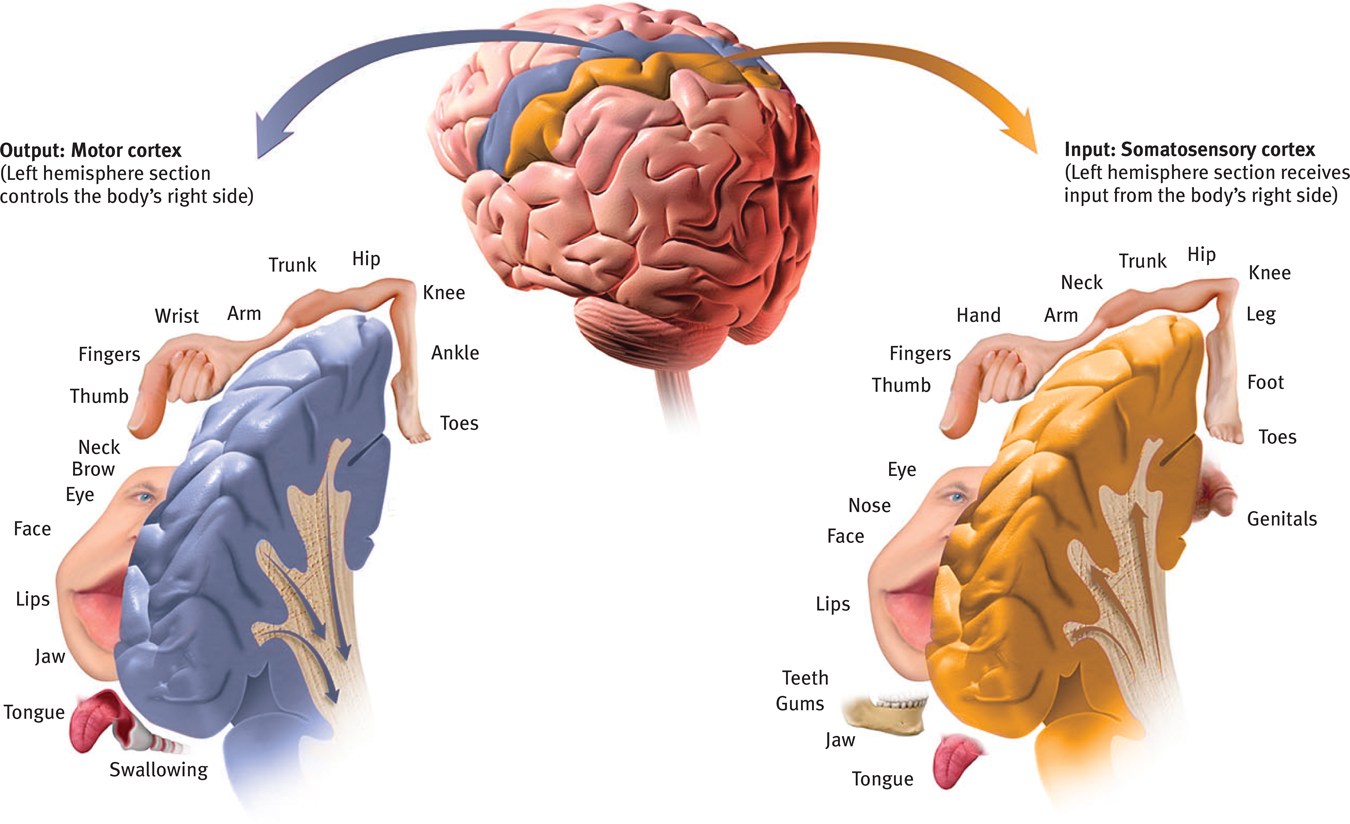
 Figure 7.2
Figure 7.2Left hemisphere tissue devoted to each body part in the motor cortex and the somatosensory cortex As you can see from this classic though inexact representation, the amount of cortex devoted to a body part in the motor cortex (in the frontal lobes) or in the somatosensory cortex (in the parietal lobes) is not proportional to that body part’s size. Rather, the brain devotes more tissue to sensitive areas and to areas requiring precise control. Thus, the fingers have a greater representation in the cortex than does the upper arm.
RETRIEVAL PRACTICE
- Try moving your right hand in a circular motion, as if cleaning a table. Then start your right foot doing the same motion, synchronized with your hand. Now reverse the right foot’s motion, but not the hand’s. Finally, try moving the left foot opposite to the right hand.
- Why is reversing the right foot’s motion so hard?
- Why is it easier to move the left foot opposite to the right hand?
1. The right limbs’ opposed activities interfere with each other because both are controlled by the same (left) side of your brain. 2. Opposite sides of your brain control your left and right limbs, so the reversed motion causes less interference.
More recently, scientists were able to predict a monkey’s arm motion a tenth of a second before it moved—
BRAIN–COMPUTER INTERFACES By eavesdropping on the brain, could we enable a paralyzed person to move a robotic limb? Could a brain–
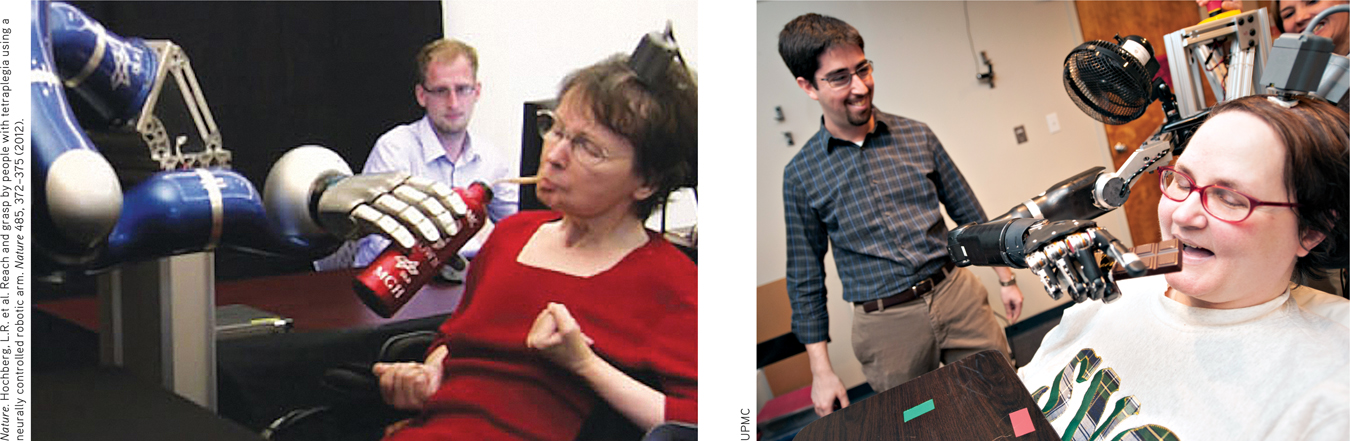
 Figure 7.3
Figure 7.3Mind over matter Strokes caused Cathy’s (left) complete paralysis, as did a neurodegenerative disease for Jan (right). Yet, thanks to a tiny, 96-
Research has also recorded messages not from the arm-
If this technique works, why not use it to capture the words a person can think but cannot say (for example, after a stroke)? Cal Tech neuroscientist Richard Andersen (2004, 2005) has speculated that researchers could implant electrodes in speech areas, then “ask a patient to think of different words and observe how the cells fire in different ways. So you build up your database, and then when the patient thinks of the word, you compare the signals with your database, and you can predict the words they’re thinking. Then you take this output and connect it to a speech synthesizer. This would be identical to what we’re doing for motor control.” With this goal in mind, the U.S. Army is investing $6.3 million in neuroscientists’ efforts to build a helmet that might read and transmit soldiers’ thoughts (Piore, 2011).
Clinical trials of such cognitive neural prosthetics are now under way with people who have suffered paralysis or amputation (Andersen et al., 2010; Nurmikko et al., 2010). The first patient, a paralyzed 25-
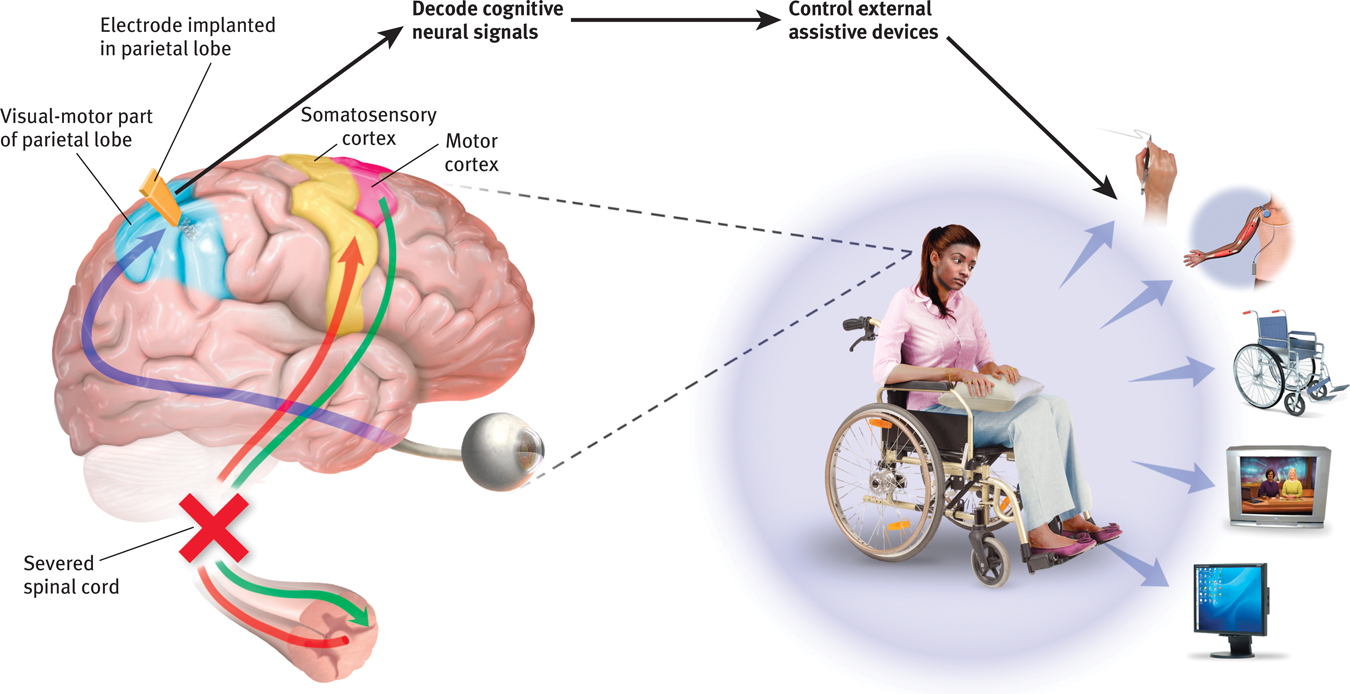
 Figure 7.4
Figure 7.4Brain–
Sensory FunctionsIf the motor cortex sends messages out to the body, where does the cortex receive incoming messages? Penfield identified a cortical area—
The more sensitive the body region, the larger the somatosensory cortex area devoted to it (Figure 7.2). Your supersensitive lips project to a larger brain area than do your toes, which is one reason we kiss with our lips rather than touch toes. Rats have a large area of the brain devoted to their whisker sensations, and owls to their hearing sensations.
Scientists have identified additional areas where the cortex receives input from senses other than touch. Any visual information you are receiving now is going to the visual cortex in your occipital lobes, at the back of your brain (FIGURES 7.5 and 7.6). Stimulated in the occipital lobes, you might see flashes of light or dashes of color. (In a sense, we do have eyes in the back of our head!) Having lost much of his right occipital lobe to a tumor removal, a friend was blind to the left half of his field of vision. Visual information travels from the occipital lobes to other areas that specialize in tasks such as identifying words, detecting emotions, and recognizing faces.
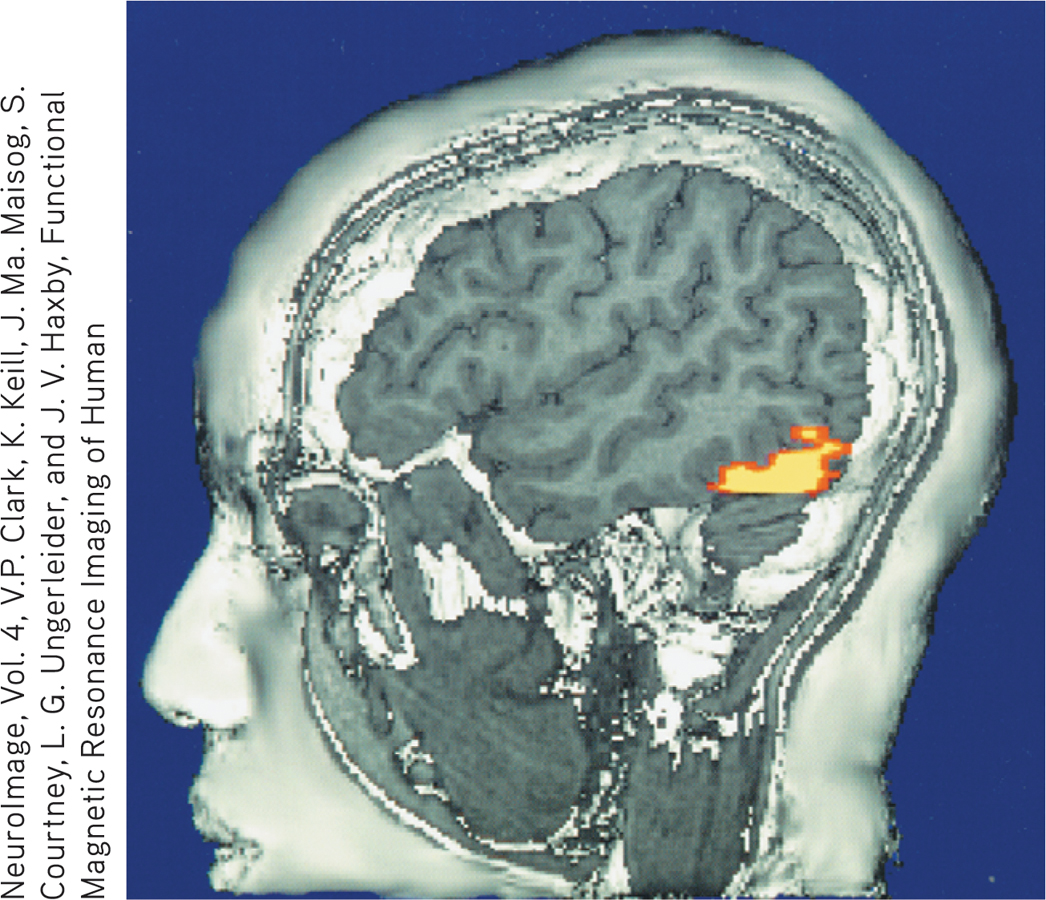
 Figure 7.5
Figure 7.5The brain in action This fMRI (functional MRI) scan shows the visual cortex in the occipital lobes activated (color represents increased bloodflow) as a research participant looks at a photo. When the person stops looking, the region instantly calms down.
Any sound you now hear is processed by your auditory cortex in your temporal lobes (just above your ears; see Figure 7.6). Most of this auditory information travels a circuitous route from one ear to the auditory receiving area above your opposite ear. If stimulated in your auditory cortex, you might hear a sound. MRI scans of people with schizophrenia have revealed active auditory areas in the temporal lobes during the false sensory experience of auditory hallucinations (Lennox et al., 1999). Even the phantom ringing sound experienced by people with hearing loss is—
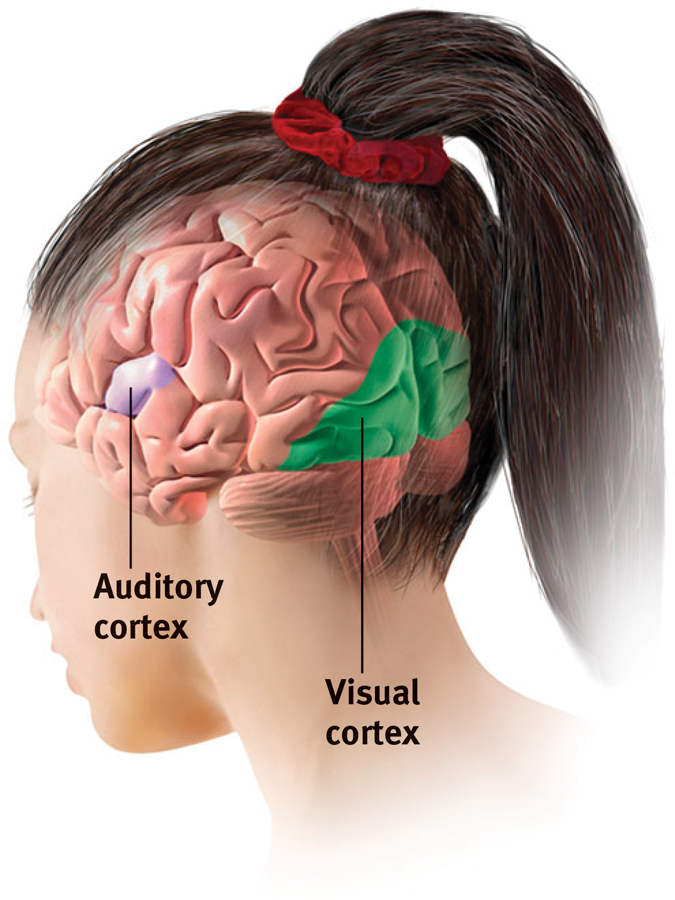
 Figure 7.6
Figure 7.6The visual cortex and auditory cortex The visual cortex in the occipital lobes at the rear of your brain receives input from your eyes. The auditory cortex, in your temporal lobes—
RETRIEVAL PRACTICE
- Our brain’s ____________cortex registers and processes body touch and movement sensations. The _____________cortex controls our voluntary movements.
somatosensory; motor
Association AreasSo far, we have pointed out small cortical areas that either receive sensory input or direct muscular output. Together, these occupy about one-
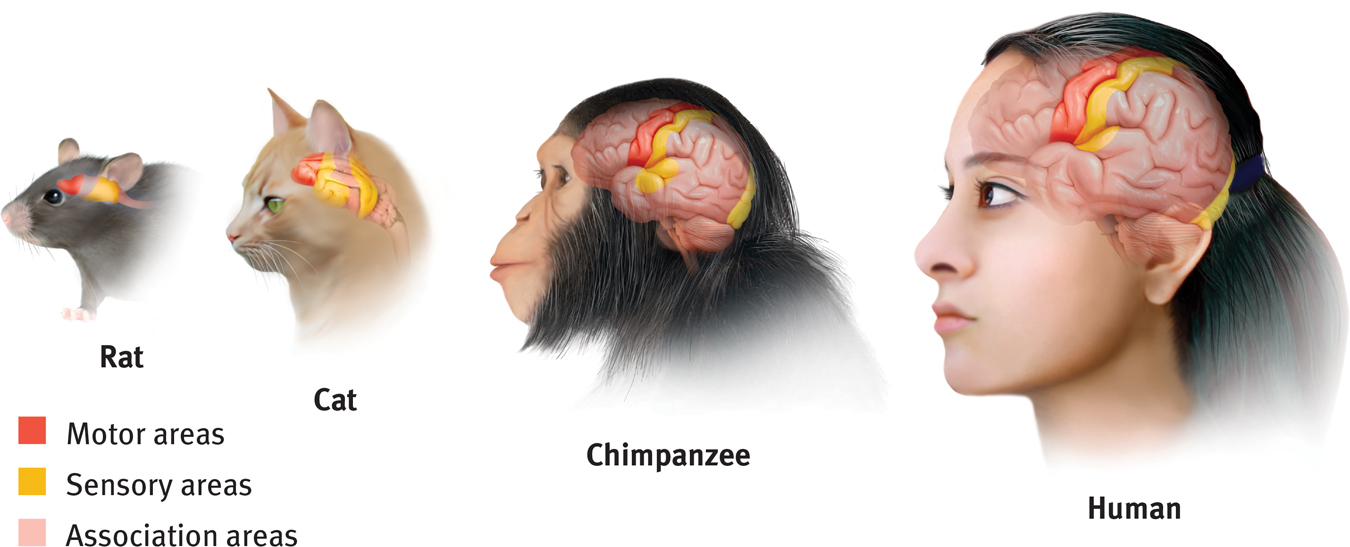
 Figure 7.7
Figure 7.7Areas of the cortex in four mammals More intelligent animals have increased “uncommitted” or association areas of the cortex. These vast areas of the brain are responsible for interpreting, integrating, and acting on sensory information and linking it with stored memories.
Electrically probing an association area won’t trigger any observable response. So, unlike the somatosensory and motor areas, association area functions cannot be neatly mapped. Their silence has led to what Donald McBurney (1996, p. 44) called “one of the hardiest weeds in the garden of psychology”: the claim that we ordinarily use only 10 percent of our brain. (If true, wouldn’t this imply a 90 percent chance that a bullet to your brain would strike an unused area?) Surgically lesioned animals and brain-
Association areas are found in all four lobes. The prefrontal cortex in the forward part of the frontal lobes enables judgment, planning, and processing of new memories. People with damaged frontal lobes may have intact memories, high scores on intelligence tests, and great cake-
Frontal lobe damage also can alter personality and remove a person’s inhibitions. Consider the classic case of railroad worker Phineas Gage. One afternoon in 1848, Gage, then 25 years old, was using a tamping iron to pack gunpowder into a rock. A spark ignited the gunpowder, shooting the rod up through his left cheek and out the top of his skull, leaving his frontal lobes damaged (FIGURE 7.8). The rod not only damaged some of Gage’s left frontal lobe’s neurons, but also about 11 percent of its axons that connect the frontal lobes with the rest of the brain (Van Horn et al., 2012). To everyone’s amazement, he was immediately able to sit up and speak, and after the wound healed he returned to work. But having lost some of the neural tracts that enabled his frontal lobes to control his emotions, the affable, soft-
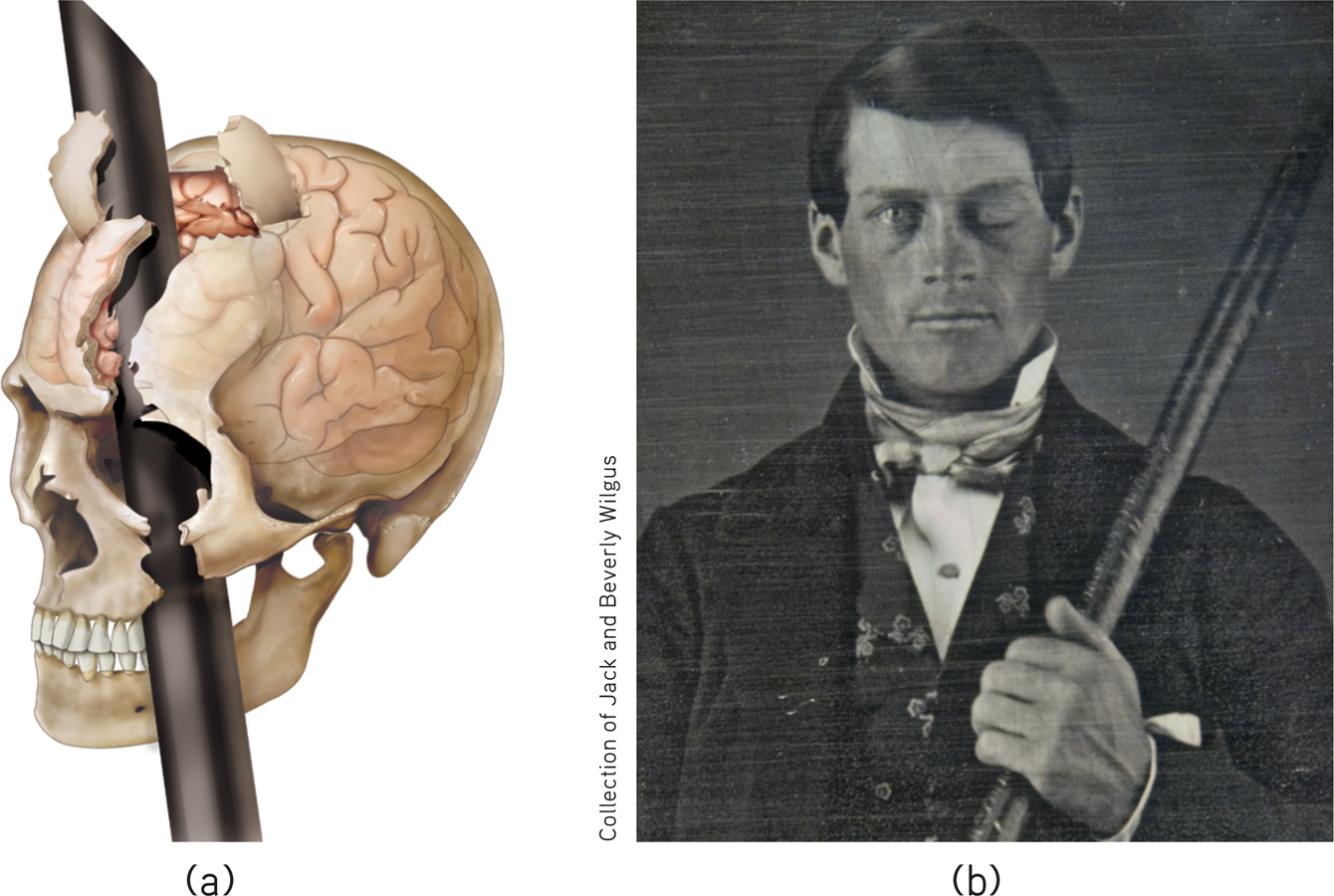
 Figure 7.8
Figure 7.8A blast from the past (a) Phineas Gage’s skull was kept as a medical record. Using measurements and modern neuroimaging techniques, researchers have reconstructed the probable path of the rod through Gage’s brain (Van Horn et al., 2012). (b) This photo shows Gage after his accident. (The image has been reversed to show the features correctly. Early photos, including this one, were actually mirror images.)
Studies of others with damaged frontal lobes have revealed similar impairments. Not only may they become less inhibited (without the frontal lobe brakes on their impulses), but their moral judgments may seem unrestrained by normal emotions. Would you advocate pushing one person in front of a runaway trolley to save five others? Most people do not, but those with damage to a brain area behind the eyes often do (Koenigs et al., 2007). With their frontal lobes ruptured, people’s moral compass seems to disconnect from their behavior.
Association areas also perform other mental functions. The parietal lobes, parts of which were large and unusually shaped in Einstein’s normal-
On the underside of the right temporal lobe, another association area enables us to recognize faces. If a stroke or head injury destroyed this area of your brain, you would still be able to describe facial features and to recognize someone’s gender and approximate age, yet be strangely unable to identify the person as, say, your grandmother.
Nevertheless, as noted elsewhere, we should be wary of using pictures of brain “hot spots” to create a new phrenology that locates complex functions in precise brain areas (Beck, 2010; Shimamura, 2010; Uttal, 2001). Complex mental functions don’t reside in any one place. There is no one spot in a rat’s small association cortex that, when damaged, will obliterate its ability to learn or remember a maze. Your memory, language, and attention result from the synchronized activity among distinct brain areas and neural networks (Knight, 2007). Ditto for religious experience. More than 40 distinct brain regions become active in different religious states, such as prayer and meditation, indicating that there is no simple “God spot” (Fingelkurts & Fingelkurts, 2009). The point to remember: Our mental experiences arise from coordinated brain activity.
RETRIEVAL PRACTICE
- Why are association areas important?
Association areas are involved in higher mental functions—
The Brain's Plasticity
7-
Our brains are sculpted not only by our genes but also by our experiences. MRI scans show that well-
Some brain-
Plasticity may also occur after serious damage, especially in young children (Kolb, 1989; see also FIGURE 7.9). Constraint-
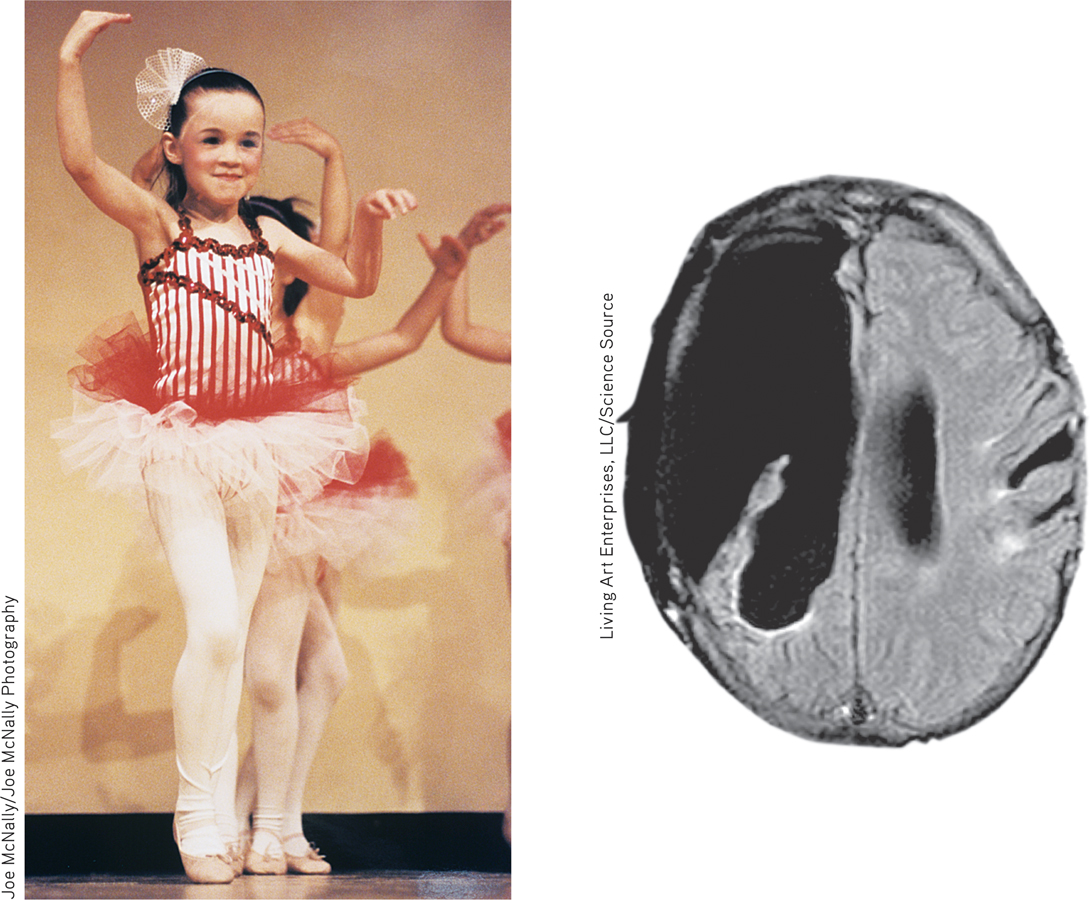
 Figure 7.9
Figure 7.9Brain plasticity This 6-
The brain’s plasticity is good news for those blind or deaf. Blindness or deafness makes their unused brain areas available for other uses (Amedi et al., 2005). If a blind person uses one finger to read Braille, the brain area dedicated to that finger expands as the sense of touch invades the visual cortex that normally helps people see (Barinaga, 1992a; Sadato et al., 1996). Plasticity also helps explain why some studies have found that deaf people have enhanced peripheral and motion-
Similar reassignment may occur when disease or damage frees up other brain areas normally dedicated to specific functions. If a slow-
Although the brain often attempts self-
Cold War nuclear tests between 1945 and 1963 oddly later enabled scientists to confirm the birth of new brain neurons. The blasts released radioactive carbon isotopes, which carbon-
Master stem cells that can develop into any type of brain cell have also been discovered in the human embryo. If mass-