Chapter 1. LAB 11 Molecular Techniques 2: Fingerprinting DNA
Introduction
Learning Goals
- Define DNA fingerprint and understand how DNA fingerprints can be produced through restriction enzyme digestions
- Understand how agarose gel electrophoresis allows for the resolution of DNA fragments of differing sizes
- Know the steps necessary to solve the case of the alleged “nosocomial infection” using DNA fingerprinting
- Know how to retrieve nucleotide sequences from the NCBI GenBank database and use scientific software, such as NEBcutter, to produce DNA fingerprints made by specific restriction enzymes
- Understand how to read a gel picture of DNA fragments and estimate fragment length using a DNA marker and migration distances
Lab Outline
Activity 2: Practicing Loading and Running Gels
Activity 3: DNA Virtual Gel Worksheet
Activity 4: NEBcutter and Plasmid Strip Comparison
Scientific Inquiry
What do CSI, Bones, and NCIS have in common? They are television series that showcase forensic science—a field of science that uses many different scientific techniques to investigate legal issues. The popular media has capitalized on the wonders of forensic methods ever since DNA fingerprinting became an important source of court evidence in the early 1980s. Although forensic science itself is a rather old discipline (predating popular characters such as Sherlock Holmes), it was not until the dawn of DNA technology that it became a mainstream topic.
DNA fingerprinting was invented by a geneticist, Dr. Alex Jeffreys. In the late 1970s, Dr. Jeffreys was interested in detecting variations within human DNA. He began with the β-globin gene family because it was one of the few human genes characterized at the time. Now, about 35 years later, every human gene has been sequenced and mapped to a particular chromosome, but when Dr. Jeffreys started his work, very little was known about DNA sequences.
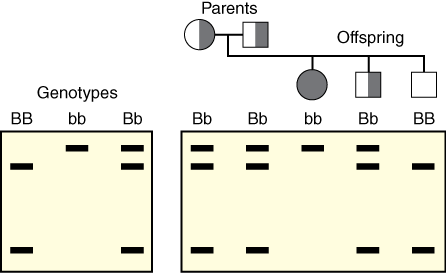
Dr. Jeffreys used restriction endonucleases (enzymes that cut DNA) and agarose gel electrophoresis to show variations in DNA lengths called restriction fragment length polymorphisms (RFLPs) at the β-globin locus (Jeffreys 2005). Some alleles of genes at the β-globin locus gave one pattern of DNA fragments, while other alleles from the same locus gave a slightly different pattern, due to changes in the size of the restriction fragments produced (Figure 1). However, these RFLPs did not work well for characterizing differences in alleles between humans.
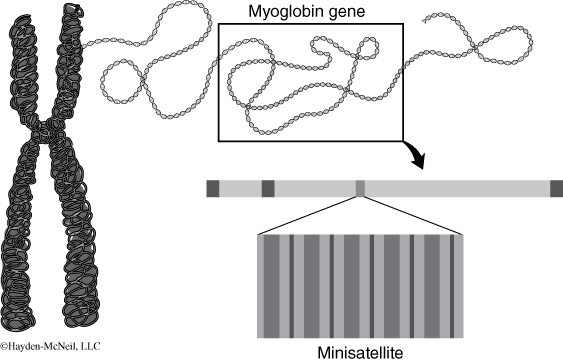
This led to the search for and discovery of genetic markers called minisatellite DNA. Minisatellites are short stretches of a repeating sequence (usually 10–30 base pairs) that occur in tandem (one right after the other) in many regions of the human genome (Figure 2). While the number of these repeats is highly variable, this variability is inheritable so that family members can easily be distinguished from non-family members. The pattern of these minisatellites that is produced after restriction digestion and agarose gel electrophoresis allows one to distinguish different individuals. Again, the pattern of these minisatellites produced after restriction digestion and agarose gel electrophoresis allows one to distinguish different individuals,using DNA samples only. The implications of this discovery were staggering.
The first test of this new technology involved an immigration case. A lawyer requested Dr. Jeffreys’ help in providing evidence that a young man from Africa was actually a woman’s son so that he would not be deported. Traditional blood tests showed that the woman and the boy were related, but could not show how closely they were related. Dr. Jeffreys was able to use these minisatellite markers and show that the woman was significantly more related to the boy than an aunt would be and the boy retained his citizenship (Jeffreys 2005). Subsequently, Dr. Jeffreys solved a double murder rape case in Leicestershire, England. DNA samples were collected from men in all the local pubs in the area. The original suspect was released because there was no match between his DNA and the DNA from the semen left at the scene. This was the first time that DNA was used to vindicate someone. The ability of DNA evidence to exonerate an innocent person spurred the formation of the Innocence Project in the United States in the 1990s.
Although DNA fingerprinting has advanced considerably through the invention of PCR and development of better DNA markers, the simple process of isolating DNA and mapping it with restriction enzymes and agarose gel electrophoresis that Dr. Jeffreys discovered is not outdated. These techniques are still commonly used even in the most sophisticated of molecular biology laboratories and they are still used forensically.
For example, if a bacterial infection breaks out at a hospital, and if the infection confers resistance to a particular antibiotic, it is most likely due to plasmid exchange between bacteria, since the plasmid usually contains genes that confer antibiotic resistance for its host bacteria. The bacteria are cultured and the plasmid DNA is isolated and digested with restriction enzymes to produce a plasmid fingerprint (Figure 3). If all of the plasmids isolated from the different infections produce the same restriction pattern, then the infection most likely originated from the same source and the origin of this source can be tracked with more advanced methods.
During this lab, you will work to solve a fictitious case using the same forensic analysis techniques that scientists use. You will also learn molecular biology approaches used to analyze recombinant DNA molecules. To this end, you will start with small rings of DNA called plasmids that have already been isolated from bacterial cells. Plasmids are vectors commonly used for cloning genes, and most cloning procedures use the bacterium E. coli. A second important procedure in this technology is the digestion of plasmid DNA into manageable pieces with the use of restriction enzymes. Once your plasmid DNA is cut, you will size-sort the fragments using gel electrophoresis and actually see the fragments in the gel using special stains. Gel electrophoresis allows you to sort DNA molecules by size and see the fragment patterns produced by restriction enzyme digestion.
Although plasmid vectors can be a source of DNA for forensic analysis, these rings of DNA can also be cut open with restriction enzymes, fused with a foreign piece of DNA, and re-sealed with ligase, recreating a new and larger plasmid ring of recombinant DNA. This is gene-splicing, a technique for introducing new genes into a plasmid so the gene product can be produced by a host bacterium. Recombinant DNA molecules are inserted into a new host cell (usually E. coli) in a process called transformation. Transformation in a lab is the same process that occurs naturally in bacteria in the wild—plasmids are transferred from one bacterium to another with their genes, which often contain genes for antibiotic resistance.
In this lab, you will analyze information that will aid in a case of alleged harm due to a hospital-induced, or nosocomial, infection (Singh et al. 2006). The lawyer for the defendants states that their young daughter entered the hospital for a relatively simple operation. Her stay was prolonged due to the development of a bacterial infection that caused grave complications and required an additional surgery that was only partially covered by her parents’ insurance. The hospital is the plaintiff in this case, demanding full payment for the additional surgery. The parents believe the infection was nosocomial and report that there was a rash of such occurrences during their daughter’s stay. They report that everyone on their daughter’s ward had to gown up and wear masks before they entered this particular hospital ward. The hospital claims that the young girl’s infection is not related to that of any of the other patients and the mask and gowns were a precaution to prevent any spread of resistant bacteria.
Four different patients (A, B, C, and D) from her ward volunteered to be tested to determine if there was a common source of infection at the hospital. Your group will complete the forensic analyses for two of these samples (A and B, A and C, A and D, B and C, B and D, or C and D) to determine whether the plasmids isolated from the infectious bacteria match the young girl’s sample. If your team can show that there is at least one other patient match, the court will mandate testing of samples from all the patients on that hospital ward.
Background
Restriction Enzymes
Restriction enzymes, also known as restriction endonucleases, catalyze double-stranded breaks in DNA molecules. You can think of them as very specific “DNA scissors,” where each restriction enzyme binds to specific “recognition sequences” of nucleotides at cleavage sites. The recognition sequence for each restriction enzyme is palindromic, which means that it reads the same forward as it does backwards. Since DNA is double-stranded, the sequence reads 5′ to 3′ on the top strand the same as it does 5′ to 3′ on the bottom, or complementary, strand.
For example, the recognition sequence for the restriction enzyme PvuII is CAGCTG (Figure 4).
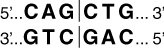
It reads CAGCTG going 5′ to 3′ on the top DNA strand and it reads CAGCTG when going 5′ to 3′ on the bottom DNA strand.
When PvuII cuts DNA, it cuts straight across from one DNA strand to the other—thus it makes a blunt cut. It cuts the phosphodiester bond between the G and the C going 5’ to 3’ on the top DNA strand and between the G and the C on the complementary strand. Not all restriction enzymes cut DNA this way. Some, such as BamHI with the recognition sequence G^GATCC, make staggered cuts in the DNA leaving what are known as sticky ends (Figure 5).

Any DNA molecule cut with BamHI can be joined together with any other DNA molecule cut with BamHI because the bases at the end of each cut will pair with each other. Restriction enzymes that leave cohesive, or sticky, ends are restricted to pairing sticky ends with matching base pairs, whereas blunt ends cut with enzymes, such as PvuII, can match to any other DNA fragment with a blunt end, regardless of the enzyme that cut it. So, while ends produced by the restriction endonuclease BamHI are not compatible with ends produced by that of HindIII (another restriction enzyme that leaves sticky ends), ends produced by the two blunt cutters PvuII and EcoRV are compatible. The covalent joining of two different DNA molecules into a single continuous DNA molecule is the basis for recombinant DNA or cloning technology.
Agarose Gel Electrophoresis
DNA samples that have been digested using restriction enzymes usually contain a mixture of many billions of DNA fragments of different sizes. These fragments can be separated by size using a technique known as agarose gel electrophoresis, which not only separates DNA fragments by size, but also allows their visualization with the use of special dyes (Figure 6). Gel electrophoresis exploits the fact that DNA is negatively charged due to its phosphate groups—therefore DNA will naturally be drawn towards the positive pole in an electric field. The agarose gel is a porous semi-solid that acts as a selective molecular sieve allowing smaller DNA fragments to pass through it more quickly than larger DNA fragments.
DNA samples mixed with loading dye are added to the wells at one end of the gel. An electrical current is applied across the gel which drives the negatively charged DNA fragments away from the negatively charged cathode end of the gel towards the positively charged anode end of the gel—the shorter DNA fragments travel farther than the longer fragments.
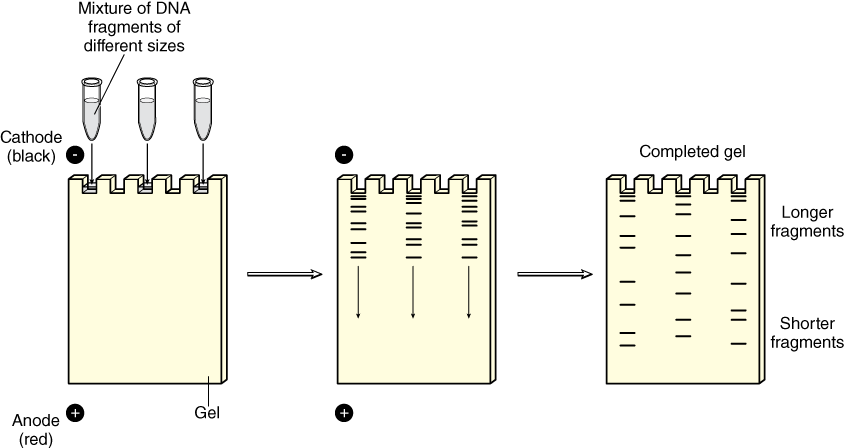
In preparation for electrophoresis, the agarose gel is immersed in a buffer. Loading dye is added to the DNA samples before they are injected into the wells of the agarose gel. Loading dye contains a viscous agent that keeps the DNA sample in the gel wells so it does not float out into the buffer. The loading dye also contains colored dyes that migrate similarly to certain DNA fragment sizes so we can tell how far the invisible DNA has travelled through the gel. When a voltage current is applied across the gel, the DNA fragments migrate through it from the negative towards the positive electrode and sort by size as they go. The longer (larger) DNA fragments reptate, or “snake,” through the porous gel material more slowly than shorter fragments.
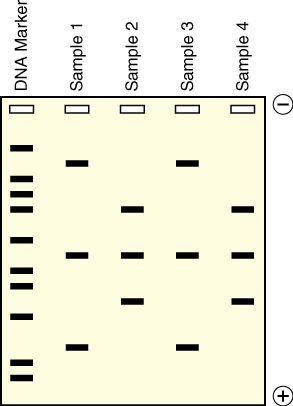
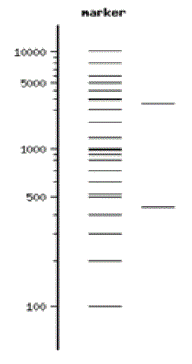
DNA itself is not visible during the gel run unless a fluorescent dye has been added to the agarose gel during its preparation and the gel is exposed to UV light. These dyes “squeeze” between the DNA bases and fluoresce green, orange, or gold when exposed to UV light depending on the type of dye used. The pattern of sorted DNA fragments on your virtual gel should correspond to the DNA fragment sizes predicted by your restriction digestion calculations. You can record results by taking pictures of the DNA fragments with conventional Polaroid photography or by digital imaging. One lane of a gel will contain a DNA marker that is made up of a mixture of DNA fragments of known sizes. The size of each unknown DNA fragment can be estimated by comparing it to the known sizes of the fragments in the DNA marker run in an adjacent lane of the gel (Figure 7).
You will analyze plasmids isolated from the bacterial infections of patients A, B, C, and D and determine if they produce restriction patterns identical to that of the plasmid DNA sample from the bacteria isolated from the defendant. Would you expect identical DNA molecules to produce the same restriction map for a given restriction enzyme?
Resources
1.1 Lab Preparation
Lab Preparation
Watch the vodcast and read this lab. Write all notes in your lab notebook. Complete the “Use of Software in Science” pre-lab assignment.
1.2 Use of Software in Science
(Complete this assignment prior to lab)
Learning Objectives
After successful completion of this activity, you should be able to:
LO112 Label the sizes of standard bands in the DNA marker based on a key
Use of scientific software programs for planning experiments and analyzing data is integral to the modern research process. If you plan to clone a gene, there are programs to help you determine which restriction enzymes would be best to use. But you need to know 1) the sequence of the gene and 2) the sequence of the plasmid that you plan to insert the gene into. You may be able to retrieve the gene sequence from the public government database at NCBI called GenBank. If the plasmid was purchased from a biological company, then you can probably copy and paste the sequence from their website.
Once you know the sequence of your DNA, you can use NEBcutter, software that is freely accessible on the Web, to produce a restriction map of your DNA. A restriction map is a list of every enzyme that you designate and the exact position it cuts your DNA. If you know the GenBank accession number for your DNA (this is the number whereby every DNA molecule in GenBank is cataloged), then NEBcutter will retrieve the DNA sequence for you. If you are interested in the restriction map of a particular piece of DNA for a given enzyme, then this software is an easy way to produce a virtual gel run of that result.
Prepare for this lab by performing a virtual digestion and agarose gel electrophoresis of a plasmid called pMC524/MBM. This plasmid was isolated during the study of multi-drug resistant nosocomial Staphylococcus aureus (Bhakta and Bal 2003). This plasmid also replicates in E. coli.
- Access NEBcutter by going online and searching for the site or type in the URL http://tools.neb.com/ and click on the link for the software.
- Type in the pMC524/MBM GenBank number AJ312056 in the space provided (most sequence software, whether free or for purchase, are linked to this site). Optionally, you can copy and paste the sequence for pMC524/MBM from GenBank or Blackboard into the box. If a sequence you are working with is not in GenBank, then you will need to copy and paste it into NEBcutter.
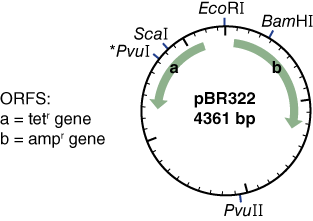
- Select “circular” because it is a plasmid and “all commercially available specificities” because you are only interested in enzymes that you can purchase if necessary. Leave the ORF (Figure 8) number at 100.
- Type the plasmid’s name in the space provided where it says “Name of sequence” (optional), otherwise the program will use the GenBank number as the name of the DNA molecule.
- Click Submit. What happens, and how is the sequence displayed?
- Save the page to a memory stick as NEBmap pMC524. You can do this in one of the following ways. Save as a webpage by going to the File menu on the web browser and selecting “save as” or “save page as.” You can also save it in PDF format by going to the Print menu under File and selecting Adobe PDF as the printer. You will be prompted to name the file.
- Digest pMC524/MBM with PvuII by clicking on Custom Digest in the Main options panel.
- In the box next to Pick this enzyme, type pvuii (remember that the II in PvuII is Roman numeral I or i in the enzyme name). Click OK.
- Scroll down the page and look at the box next to PvuII. Is it checked? Could you have checked the box rather than write in the name of the enzyme?
- Digest the plasmid by clicking on “digest” in the menu. Explain what happens in your lab notebook. Save the picture as NEBmap pMC524 PvuII.
- Click View gel in the Main options menu. This software will produce a virtual gel, allowing you to visualize the fragments produced from digestion of pMC524/MBM with PvuII. Select the % agarose gel from the drop-down menu based on what you will use in the lab. Select the 2-log DNA ladder as your marker from the drop-down menu. Save the page to your memory stick as NEBgel pMC524 PvuII 2log.
- Look at the difference in migration of the DNA fragments on different percentage gels (0.7%, 1%, 2%, and 3%). What do you notice about the effect on the migration and separation of the DNA fragments, especially those in the marker? What does increasing the % agarose appear to do?
- Now repeat this process (virtual digestion of pMC524/MBM) with the restriction enzyme EcoRI by selecting New custom digests under Main options. Try other enzymes such as HindIII or SspI.
- In what way(s) is software like NEBcutter a valuable tool for a scientist as well as for undergraduate students?
- Print out a copy of your saved files and bring them to lab with you.
- If the DNA sequences of the plasmids from bacteria isolated from patients A, B, C, and D are available on Blackboard, how can you use NEBcutter to make restriction pattern predictions for these DNA samples?
Before starting any Lab 11 activities, please complete Lab 13 Activities 1 and 2.
Activity 1: Exploring DNA with the DNA Discovery Kit
In this activity your group will cooperate with the rest of the class to build a DNA model. You will also simulate the plectonemic nature of double-stranded DNA using toobers.
Learning Objectives
After successful completion of this activity, you should be able to:
LO110 Explain the different types of DNA in bacteria (plasmids vs. chromosomal)
Nucleotides and Polynucleotides
- As a group, take one of each of the four nucleotides A, T, C, and G from the DNA Discovery Kit. Compare them; which parts of each of the nucleotides are the same and which are different?
- Review the names and locations of the major components that make up a nucleotide. Compare the appearance of a picture of the nucleotide to the model of the nucleotide (Figure 9). In what ways does the picture of the nucleotide look different from the model of the nucleotide? What kind of bond joins the phosphate to the sugar? What kind of bond joins the base to the sugar?
- Join all possible combinations of two nucleotides together as a single strand of DNA (i.e., do not base pair the nucleotides). How many possible dinucleotides can be formed?
- What is the name of the bond that forms between the two nucleotides and what chemical groups are involved in the bonding?
- Now add the other two nucleotides to this pair and construct a tetra (four) nucleotide single-stranded DNA molecule. How many possible tetranucleotides can be formed and what implications, if any, does this have for encoding genetic information?
- How stable do you think a single-stranded polynucleotide is? How easy do you think it would be to break it up into separate nucleotides or into its components of phosphates, sugars, and bases? Notice the orientation of one nucleotide with respect to the next one as you build the polynucleotide (single-stranded DNA). What overall shape does the polynucleotide take? Are both ends the same or different?
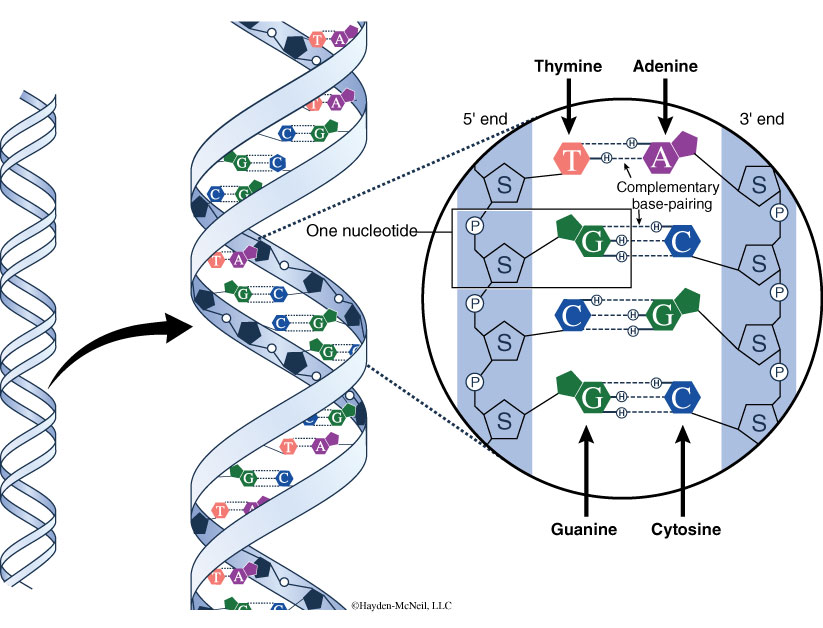
Building a Double-Stranded DNA Helix
- Examine the bases in the DNA Discovery Kit. Notice that in addition to the place where the base can be joined with the sugar, there are also magnets on the red oxygen, blue nitrogen, and white hydrogen. They represent the tendency of these atoms to form “hydrogen bonds”—O with H–N, and N with H–N or H–O.
- Now make base pairs between all the possible bases (purines–purines, pyrimidines–pyrimidines, purines–pyrimidines). Do you discover 12 non-redundant pairings? How many of these base pairs hydrogen-bonded using this model?
- Can all these possible nucleotide pairs be used to construct a double-stranded segment of DNA? If not, which ones can? How many different sequences can you construct using six nucleotides?
- Can you construct a segment of double-stranded DNA in which the backbone of both strands run anti-parallel to one another? How about parallel to one another?
- Try constructing a double-stranded DNA molecule on the model stand. Begin by placing your group’s hydrogen-bonded nucleotides on the stand. Each group will add their base pairs one after the other. Your DNA molecule should begin to look like a spiral staircase of polynucleotides, with the nucleotides hydrogen-bonded to each other forming base pairs.
Exploring the Structure of Double-Stranded DNA
- Do the nucleotides in the double-stranded DNA helix follow Chargaff’s rule?
- What is holding the two DNA helices or strands together?
- Are the sugar and phosphate groups present on the inside or outside of the helices? How is a nucleotide in one helix connected to a nucleotide adjacent to it in the same helix? Is this what you observed when you made single-stranded polynucleotides earlier? What bond connects the sugar in one nucleotide to the phosphate in the adjacent nucleotide?
- Follow the sugar–phosphate backbone along one helix. How do the paths of the two helices compare to one another if you traverse each helix going from phosphate to sugar to phosphate to sugar in the backbone? Which helix is oriented 5′ to 3′ traveling top to bottom, and which helix is oriented 5′ to 3′ traveling bottom to top? Hint: The 5′ end of the sugar has the phosphate group attached to it. Why is an understanding of DNA orientation important? Hint: Is there a particular way in which DNA is replicated? Transcribed?
- Move your right hand upward along the double-stranded DNA helix model. Does the double-stranded helix curve to the right or to the left? If it curves to the right, then this DNA model forms a right-handed helix. Why should the helix orientation matter?
- How many base pairs are there in a complete 360° turn? If you place your finger at the first base pair of the DNA model and trace along the double-stranded DNA for one 360° turn with your other hand, you will find _____ base pairs per turn.
- As you follow the grooves along the side of the double-stranded DNA helix, do you notice that the groove on one side of the DNA staircase is larger than the other? These are known as the major and minor grooves of DNA. Why are the grooves unequal in size? Hint: How are the sugars positioned relative to the nucleotide bases—are they positioned in line with the bases or at an angle? What role do the grooves play? Hint: How do proteins specifically bind DNA?
- Your instructor will demonstrate the plectonemic nature of double-stranded DNA using toobers and discuss types of DNA molecules in bacteria.
Activity 2: Practicing Loading and Running Gels
In this activity, you will use pre-poured agarose gels. The gels are made by weighing solid agarose powder, placing it into a flask, adding gel electrophoresis buffer, and boiling the mixture in a microwave oven until the agarose powder dissolves. When the gel solution cools to the touch, typically a DNA-detection reagent called SYBR Safe is added to the molten agarose. The agarose is poured into a comb-containing gel mold, and allowed to cool and solidify. While you will not make your own gel, you are responsible for knowing how it is made. You will place the pre-poured gel in an electrophoresis tank, add buffer, add dye to wells in the gel, apply voltage, and observe migration of the dye.
Learning Objectives
After successful completion of this activity, you should be able to:
Materials
Power supply
Pre-poured agarose gel
10× TAE (400 mM Tris-acetate, 10 mM EDTA) buffer, pH 8.3
Horizontal electrophoresis tank with lid
Dye samples: Xylene cyanol (runs with ~4 kbp DNA fragment in 1% agarose gel) and bromophenol blue (runs with ~300 bp DNA fragment in 1% agarose gel)
Activity 2 Procedure
Running the Agarose Gel
- Note the placement of the gel in the electrophoresis tank. The wells are near the black electrode (negative).
- 1× TAE running buffer has already been added to your electrophoresis tank. Make sure the wells are covered with buffer.
- Each student should pipette 10 μl of dye sample into one well.
- Place the lid on the electrophoresis tank so that the connector covers make proper contact with the gold tank connectors.
- Attach the leads to the power supply properly. Connect the tank electrode that is nearest the wells to the negative electrode of the power supply and the tank electrode that is at the other end of the gel to the positive electrode of the power supply.
- Switch to the low voltage setting on the power supply. Turn on the power and turn the knob until the display reads 140 Volts. Make sure that you see bubbles coming up from the electrodes in the tank. If not, check your leads and lid connections. Ask for help, if needed. Please do not apply pressure to these tanks or place any objects on top of the lid as they are extremely fragile.
- Run the gel until the line of bromophenol blue dye travels to about 2–3 centimeters from the bottom of the gel (3/4 of the distance).
- While the gel is running, complete Activity 3.
ALWAYS double-check your connections. DNA will travel towards the positive (red) electrode, so make sure that the wells of the gel are near the negative (black) electrode.
ALWAYS watch the gel for the first 5 minutes of the run to ensure that the samples are migrating in the correct direction.
ALWAYS turn off the power before disconnecting the leads or removing the lid.
Activity 3: DNA Virtual Gel Worksheet
Learning Objectives
After successful completion of this activity, you should be able to:
LO113 Draw a DNA fingerprint of a plasmid DNA cut with a restriction enzyme for which the position of the sites is known
LO114 Match materials and equipment for agarose gel electrophoresis with their function
LO118 Diagram the relationship between mapping an unknown plasmid to show how to solve a case of an alleged “nosocomial infection” using DNA fingerprinting
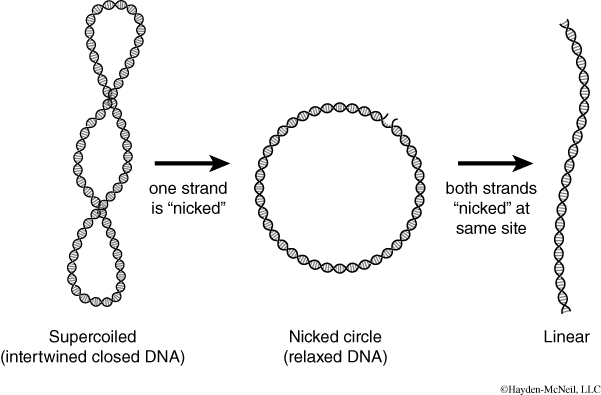
- Your instructor will give you a copy of the worksheet, which contains a gel picture of the plasmid isolated from the defendant’s bacterial infection in uncut form and after digestion with both EcoRI and PvuII. The worksheet also contains a table of the size and location of EcoRI and PvuII restriction sites for the plasmids, A, B, C, and D isolated from bacterial infections from the four patients. Remember that plasmid DNA usually exists in one of three forms: supercoiled, nicked (relaxed), and linear, which migrate at different rates in an agarose gel. Therefore, the same length of DNA can give you different bands at different distances depending upon its form (see Figure 10).
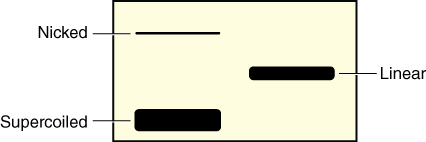
- Estimate the size of the DNA fragments on the gel picture as follows, writing necessary information in your lab notebook:
- Label the fragment sizes of your DNA standard using the marker key by going to the New England BioLabs website and searching for the 2-log DNA ladder.
- Measure the distance in millimeters that each fragment traveled, starting at the base of the wells (for the EcoRI digestions and the PvuII digestions).
- Focus on one fragment at a time and locate its position between two of the known DNA markers that the fragment of interest migrated between. Measure the distance that these known DNA fragments traveled from the well.
- Your unknown fragment traveled between these two known fragments so you can use ratios to compute the size of your unknown DNA fragment (see Box 1 for directions on how to estimate fragment size).
- Use the tabled information from your worksheet to determine the expected number of fragments for your assigned DNA samples (AB, AC, AD, BC, BD, or CD) plasmids after digestion with EcoRI and PvuII.
- Calculate the size of each of the fragments.
- For your sample 1, label the lanes on one of the schematics of a gel with 1E and 1P (for sample 1 digested with EcoRI and PvuII, respectively). For your sample 2, label the lanes in the other schematic 2E and 2P to distinguish the samples and the restriction enzymes used to digest them.
- Draw bands in each of the gel lanes relative to the 2-log DNA standard in the first lane to show the predicted migration of your DNA fragments for each digestion. Your instructor will assist you with this activity.
Activity 4: NEBcutter and Plasmid Strip Comparison
Learning Objectives
After successful completion of this activity, you should be able to:
Box 1 Estimating the Size of DNA fragments
The distance (D) that a DNA fragment migrates is inversely proportional to the log of its molecular weight (MW) or number of base pairs (bp): D = 1/log MW. In other words, the relationship is logarithmic—distance traveled on the x-axis is proportional to the inverse of the log of the molecular weight on the y-axis.
In this lab, we ask you to estimate the size of your DNA fragments using a linear scale. The linear estimation works mainly because we are comparing one fragment with the two known fragments it migrated between rather than estimating it from a standard curve that incorporates all the known fragments of your DNA marker. Let’s assume that your fragment traveled between two marker DNA fragments of 1,000 bp and 1,200 bp in size. Before you can estimate its size, you need to know the distance traveled for each fragment.
- Your unknown traveled 55 mm from the well, the 1,000 bp fragment traveled 58 mm from the well, and the 1,200 bp fragment traveled 54 mm from the well. Note that the larger the fragment, the shorter the distance it travels.
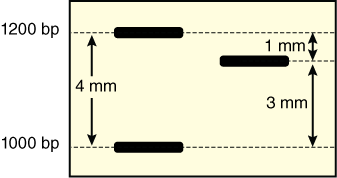
- How far is your fragment from the 1,200 bp DNA fragment in mm? From the 1,000 bp fragment in mm? Your fragment traveled 3/4th the distance between the marker fragments or 3 mm from the 1,000 bp fragment and 1 mm from the 1,200 bp fragment (Figure 11).
- How does that distance translate into base pairs? There is a 200 base pair difference between the 1,200 bp fragment and the 1,000 bp fragment over a distance of 4 mm (58 mm–54 mm) between them. Each mm therefore represents approximately 50 bp (200 bp/4 mm or 50 bp/mm).
- If the unknown fragment is 3 mm from the 1,000 bp fragment, then it is ~150 bp (3 mm * 50 bp/ mm) larger than that fragment or 1,150 bp in size. Alternatively, it is 1 mm from the 1,200 bp fragment, ~ 50 bp smaller than that fragment or 1,150 bp.
Materials
Plasmid strips for A, B, C, and D
Activity 4 Procedure
- Go to the NEBcutter website.
- Take out your two assigned plasmid strips.
- Find the sequence for the plasmids on Blackboard.
- Paste the sequence into NEBcutter.
- Complete steps 3–13 (use PvuII and EcoRI only) from the pre-lab activity Use of Software in Science.
- Where did your enzyme cut? What specific sites?
- How many fragments are there for each digestion?
- Now take out your plasmid strips (AB, AC, AD, BC, BD, or CD).
- How does the virtual gel compare to the strips?
- How does this compare to your predictions from the worksheet?