Chapter 1. Impact 10.1
Impact…ON BIOCHEMISTRY: I10.1 The reactivity of O2, N2, and NO
At sea level, air contains approximately 23.1 per cent O2 and 75.5 per cent N2 by mass. Molecular orbital theory predicts correctly that O2 has unpaired electron spins. It is a reactive component of the Earth’s atmosphere; its most important biological role is as an oxidizing agent. By contrast N2, the major component of the air we breathe, is so stable (on account of the triple bond connecting the atoms) and unreactive that nitrogen fixation, the reduction of atmospheric N2 to NH3, is among the most thermodynamically demanding of biochemical reactions, in the sense that it requires a great deal of energy derived from metabolism. So taxing is the process that only certain bacteria and archaea are capable of carrying it out, making nitrogen available first to plants and other micro-organisms in the form of ammonia. Only after incorporation into amino acids by plants does nitrogen adopt a chemical form that, when consumed, can be used by animals in the synthesis of proteins and other molecules that contain nitrogen.
The reactivity of O2, while important for biological energy conversion, also poses serious physiological problems. During the course of metabolism, some electrons reduce O2 to superoxide ion, O2–, which must be scavenged to prevent damage to cellular components. There is growing evidence for the involvement of the damage caused by reactive oxygen species (ROS), such as O2–, H2O2, and ·OH (the hydroxyl radical), in the mechanism of ageing and in the development of cardiovascular disease, cancer, stroke, inflammatory disease, and other conditions. For this reason, much effort has been expended on studies of the biochemistry of antioxidants, substances that can either deactivate ROS directly or halt the progress of cellular damage through reactions with radicals formed by processes initiated by ROS. Important examples of antioxidants are vitamin C (ascorbic acid), vitamin E (α-tocopherol), and uric acid.
Nitric oxide (nitrogen monoxide, NO) is a small molecule that diffuses quickly between cells, carrying chemical messages that help initiate a variety of processes, such as regulation of blood pressure, inhibition of platelet aggregation, and defence against inflammation and attacks to the immune system. Figure I10.1 shows the bonding scheme in NO and illustrates a number of points we have made about heteronuclear diatomic molecules. The ground configuration is 1σ22σ 23σ 21π42π1. The 3σ and 1π orbitals are predominantly of O character as that is the more electronegative element. The highest-energy occupied orbital is 2π; it is occupied by one electron and has more N character than O character. It follows that NO is a radical with an unpaired electron that can be regarded as localized more on the N atom than on the O atom. The lowest-energy unoccupied orbital is 4σ, which is also localized predominantly on N. Because NO is a radical, we expect it to be reactive. Its half-life is estimated as 1–5 s, so it needs to be synthesized often in the cell. As we saw above, there is a biochemical price to be paid for the reactivity of biological radicals.
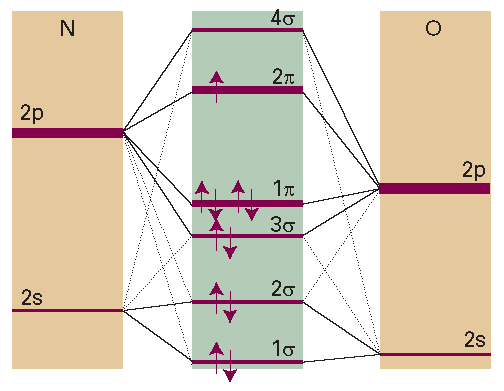
Fig. I10.1 The molecular orbital energy level diagram for NO.