Chapter 1. Impact 13.2
Impact…ON BIOCHEMISTRY: I13.2 Fluorescence microscopy
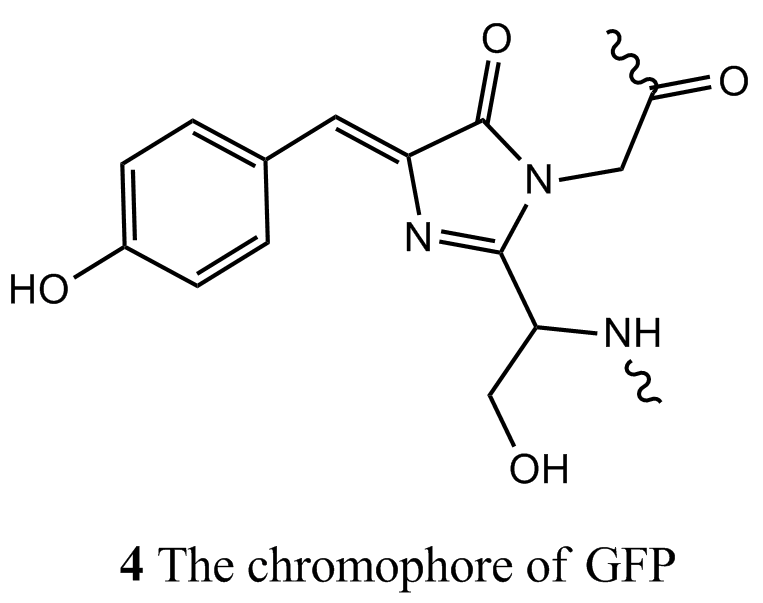
Fluorescence is a very important technique for the study of biological molecules. In fluorescence microscopy, images of biological cells at work are obtained by attaching a large number of fluorescent molecules to proteins, nucleic acids, and membranes and then measuring the distribution of fluorescence intensity within the illuminated area. Apart from a small number of co-factors, such as the chlorophylls and flavins, the majority of the building blocks of proteins and nucleic acids do not fluoresce strongly. Four notable exceptions are the amino acids tryptophan (λabs ≈ 280 nm and λfluor ≈ 348 nm in water), tyrosine (λabs ≈ 274 nm and λfluor ≈ 303 nm in water), and phenylalanine (λabs ≈ 257 nm and λfluor ≈ 282 nm in water), and the oxidized form of the sequence serine–tyrosine–glycine (4) found in the green fluorescent protein (GFP) of certain jellyfish. The wild type of GFP from Aequora victoria absorbs strongly at 395 nm and emits maximally at 509 nm and is commonly used as a fluorescent label.
Fluorescence microscopy has also been used for many years to image biological cells, but the visualization of molecules require creative strategies. In a conventional light microscope, an image is constructed from a pattern of diffracted light waves that emanate from the illuminated object. As a result, some information about the specimen is lost by destructive interference of scattered light waves. Ultimately, this diffraction limit prevents the study of samples that are much smaller than the wavelength of light used as a probe. In practice, two objects will appear as distinct images under a microscope if the distance between their centres is greater than the Airy radius, rAiry = 0.61λ/a, where λ is the wavelength of the incident beam of radiation and a is the numerical aperture of the objective lens, the lens that collects light scattered by the object. The numerical aperture of the objective lens is defined as a = nr sin α, where nr is the refractive index of the lens material (the greater the refractive index, the greater the bending of a ray of light by the lens) and the angle α is the half-angle of the widest cone of scattered light that can collected by the lens (so the lens collects light beams sweeping a cone with angle 2α).
Most molecules—including biological polymers—have dimensions that are much smaller than visible wavelengths, so special techniques had to be developed to make single-molecule spectroscopy possible. In near-field scanning optical microscopy (NSOM), a very thin metal-coated optical fibre is used to deliver light to a small area. It is possible to construct fibres with tip diameters in the range of 50 to 100 nm, which are indeed smaller than visible wavelengths. The fibre tip is placed very close to the sample, in a region known as the near field, where, according to classical physics, waves do not undergo diffraction. In far-field confocal microscopy, laser light focused by an objective lens is used to illuminate about 1 μm3 of a very dilute sample placed beyond the near field. This illumination scheme is limited by diffraction and, as a result, data from far-field microscopy have less structural detail than data from NSOM. However, far-field microscopes are very easy to construct and the technique can be used to probe single molecules as long as there is one molecule, on average, in the illuminated area.