Chapter 1. Impact 14.2
Impact…ON BIOCHEMISTRY AND NANOSCIENCE: I14.2 Spin probes
The anisotropy of the g-value and of the nuclear hyperfine interactions can be observed when a radical is immobilized in a solid. Figure I14.3 shows the variation of the lineshape of the EPR spectrum of the di-tert-butyl nitroxide radical (1) with temperature. At 292 K, the radical tumbles freely and isotropic hyperfine coupling to the 14N nucleus gives rise to three sharp peaks. At 77 K, motion of the radical is restricted. Both isotropic and anisotropic hyperfine couplings determine the appearance of the spectrum, which now consists of three broad peaks.
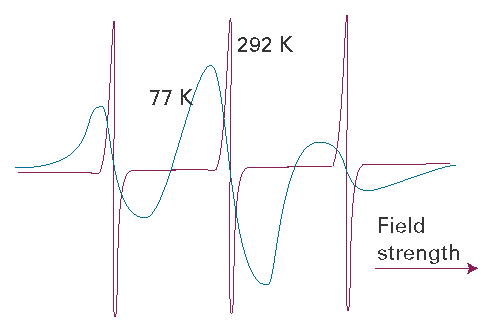
Fig. I14.3 EPR spectra of the di-tert-butyl nitroxide radical at 292 K (top) and 77 K (bottom). Adapted from J.R. Bolton, in Biological applications of electron spin resonance, H.M. Swartz, J.R. Bolton, and D.C. Borg (ed.), Wiley, New York (1972).
A spin probe (or spin label) is a radical that interacts with a molecular assembly (a biopolymer or a nanostructure) and with an EPR spectrum that reports on the structural and dynamical properties of the assembly. The ideal spin probe is one with a spectrum that broadens significantly as its motion is restricted to a relatively small extent. Nitroxide spin probes have been used to show that the hydrophobic interiors of biological membranes, once thought to be rigid, are in fact very fluid and individual lipid molecules move laterally through the sheet-like structure of the membrane. The EPR spectrum also can reveal whether a nitroxide spin probe is free in solution, positioned as a guest within a macromolecular host, or intercalated within micelles (Topic 17C). For example, hyperfine coupling constants to the 14N nucleus can change if the N–O group is exposed to the solvent or buried in the assembly.
Benzyl tert-butyl nitroxide (2) and dibenzyl nitroxide (3) are particularly well-suited spin probes for supramolecular systems. As the concentration of the host system is increased, the EPR spectrum shifts from that of the free nitroxide to that of the 1:1 complexed radical (Fig. I14.4). The variations in the nitrogen hyperfine coupling are attributed to the extent of exposure of the N–O group to water, with the lowest value for β-cyclodextrin (4) and its hydrophobic cavity. The hyperfine coupling constant for the benzyl hydrogens two bonds from the unpaired electron reflects the conformation of the nitroxide radical in the various macromolecular host systems, particularly with regard to rotation of the benzyl group about the C–N bond. The symmetric nitroxide spin probe in 3 can be incorporated into two β-cyclodextrin cavities. This 1:2 inclusion complex exhibits reduced nitrogen hyperfine splitting, which is consistent with the less polar environment achieved by the complete shielding of the nitroxide from solvent.
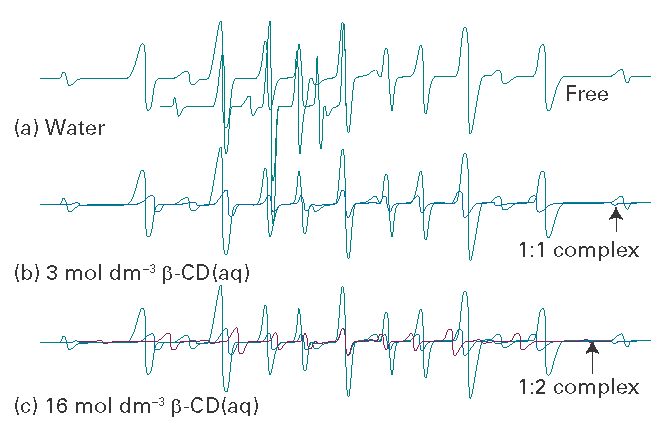
Fig. I14.4 The EPR spectra of dibenzylnitroxide in water with different concentrations of b-cyclodextrin. Based on P. Franchi, et al., Current Organic Chemistry, 1831, 8 (2004).