Chapter 1. Impact 16.2
Impact…ON MATERIALS SCIENCE: I16.2 Hydrogen storage in molecular clathrates
Hydrogen gas is efficient and environmentally clean in the sense that it is possible to use it in fuel cells without generating carbon dioxide, a greenhouse gas. Effective storage and delivery of hydrogen gas is key to the commercial development of devices that use it as a fuel. However, because H2 molecules interact only weakly with one another, the liquefaction of hydrogen for storage and transport requires very high pressures, very low temperatures, or both. For example, at 1 atm hydrogen gas condenses only at 20 K. Whereas adsorption of hydrogen on solid surfaces, a process discussed in more detail in Topic 22B, is one way to solve the storage problem, more recent solutions involve insertion of H2 molecules as guests in cage-like structurescalled clathrates.
Water is a common host, leading to solid materials known as solid hydrogen gas hydrates. One such clathrate forms at 250-600 MPa (25-60 atm) and 249 K. Hydrogen molecules are encapsulated by weak van der Waals interactions with host molecules and can be released either by increasing the temperature of by decreasing the pressure on the material.
The tetrahedral coordination geometry of the O atom in a water molecule and hydrogen bonding between water molecules lead to a variety of structures for water clathrates. Figure I16.3 shows that these structures, typically represented by adjoining polyhedra with vertices denoting the positions of O atoms, possess cages of different sizes. The so-called T cavity is a tetrakaidecahedron cage consisting of 12 pentagonal faces and two hexagonal faces. The smaller D cavity (shown in Fig. I16.3) is a pentagonal dodecahedron cage consisting of 12 pentagonal faces. The H cavity (also shown in Fig. I16.3) is a hexakaidecahedron structure consisting of 12 pentagonal and four hexagonal faces.
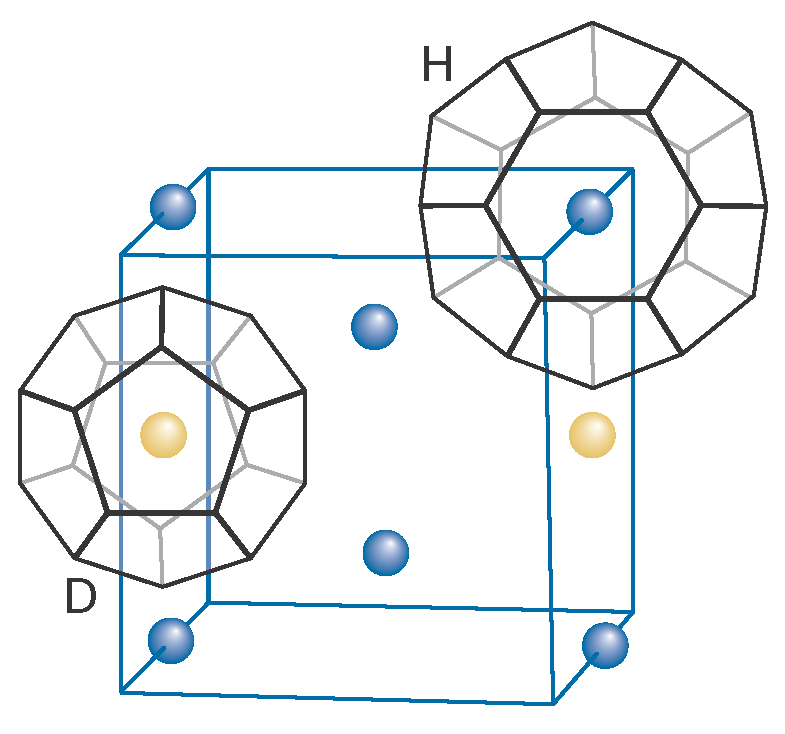
Figure I16.3 A gas hydrate showing representative D and H cavities in a partial representation of the structure. The D cavity is a pentagonal dodecahedron cage consisting of 12 pentagonal faces. The H cavity is a hexakaidecahedron structure consisting of 12 pentagonal and four hexagonal faces.
Spectroscopic and diffraction studies of hydrogen gas hydrates indicate that this type of material can encapsulate 5.2 per cent H2 by mass. This percentage is a promising level for hydrogen storage, but more research is needed to result in a material with reasonable commercial utility.