Chapter 1. Impact 22.1
Impact…ON TECHNOLOGY: I22.1 Catalysis in the chemical industry
Almost the whole of modern chemical industry depends on the development, selection, and application of catalysts (Table I22.1). All we can hope to do in this section is to give a brief indication of some of the problems involved. Other than the ones we consider, these problems include the danger of the catalyst being poisoned by byproducts or impurities, and economic considerations relating to cost and lifetime.
| Catalyst | Function | Examples |
| Metals | Hydrogenation dehydrogenation | Fe, Ni, Pt, Ag |
| Semiconducting oxides and sulfides | Oxidation desulfurization | NiO, ZnO, MgO, Bi2O3/MoO3, MoS2 |
| Insulating oxides | Dehydration | Al2O3, SiO2, MgO |
| Acids | Polymerization isomerization cracking alkylation | H3PO4, H2SO4, SiO3/Al2O3, zeolites |
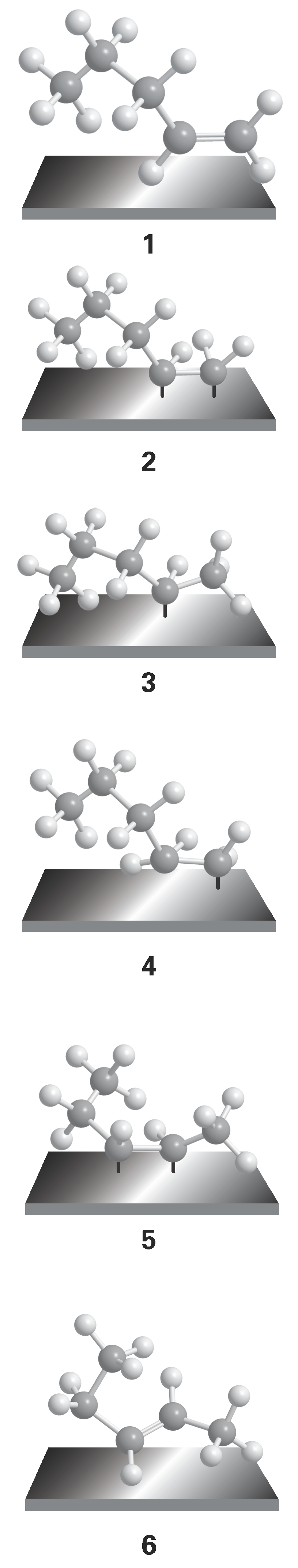
An example of catalytic action is found in the hydrogenation of alkenes. The alkene (1) adsorbs by forming two bonds with the surface (2), and on the same surface there may be adsorbed H atoms. When an encounter occurs, one of the alkene–surface bonds is broken (forming 3 or 4) and later an encounter with a second H atom releases the fully hydrogenated hydrocarbon, which is the thermodynamically more stable species. The evidence for a two-stage reaction is the appearance of different isomeric alkenes in the mixture. The formation of isomers comes about because, while the hydrocarbon chain is waving about over the surface of the metal, an atom in the chain might chemisorb again to form (5) and then desorb to (6), an isomer of the original molecule. The new alkene would not be formed if the two hydrogen atoms attached simultaneously.
A major industrial application of catalytic hydrogenation is to the formation of edible fats from vegetable and animal oils. Raw oils obtained from sources such as the soya bean have the structure CH2(OOCR)CH(OOCR′)CH2(OOCR''), where R, R', and R'' are long-chain hydrocarbons with several double bonds. One disadvantage of the presence of many double bonds is that the oils are susceptible to atmospheric oxidation, and therefore are liable to become rancid. The geometrical configuration of the chains is responsible for the liquid nature of the oil, and in many applications a solid fat is at least much better and often necessary. Controlled partial hydrogenation of an oil with a catalyst carefully selected so that hydrogenation is incomplete and so that the chains do not isomerize (finely divided nickel, in fact), is used on a wide scale to produce edible fats. The process, and the industry, is not made any easier by the seasonal variation of the number of double bonds in the oils.
Catalytic oxidation is also widely used in industry and in pollution control. Although in some cases it is desirable to achieve complete oxidation (as in the production of nitric acid from ammonia), in others partial oxidation is the aim. For example, the complete oxidation of propene to carbon dioxide and water is wasteful, but its partial oxidation to propenal (acrolein, CH2=CHCHO) is the start of important industrial processes. Likewise, the controlled oxidations of ethene to ethanol, ethanal (acetaldehyde), and (in the presence of acetic acid or chlorine) to chloroethene (vinyl chloride, for the manufacture of PVC), are the initial stages of very important chemical industries.
Some of these oxidation reactions are catalysed by d-metal oxides of various kinds. The physical chemistry of oxide surfaces is very complex, as can be appreciated by considering what happens during the oxidation of propene to propenal on bismuth molybdate. The first stage is the adsorption of the propene molecule with loss of a hydrogen to form the propenyl (allyl) radical, CH2=CHCH2. An O atom in the surface can now transfer to this radical, leading to the formation of propenal and its desorption from the surface. The H atom also escapes with a surface O atom, and goes on to form H2O, which leaves the surface. The surface is left with vacancies and metal ions in lower oxidation states. These vacancies are attacked by O2 molecules in the overlying gas, which then chemisorb as O2- ions, so reforming the catalyst. This sequence of events, which is called the Mars van Krevelen mechanism, involves great upheavals of the surface, and some materials break up under the stress.
Many of the small organic molecules used in the preparation of all kinds of chemical products come from oil. These small building blocks of polymers, perfumes, and petrochemicals in general, are usually cut from the long-chain hydrocarbons drawn from the Earth as petroleum. The catalytically induced fragmentation of the long-chain hydrocarbons is called cracking, and is often brought about on silica– alumina catalysts. These catalysts act by forming unstable carbocations, which dissociate and rearrange to more highly branched isomers. These branched isomers burn more smoothly and efficiently in internal combustion engines, and are used to produce higher octane fuels.
Catalytic reforming uses a dual-function catalyst, such as a dispersion of platinum and acidic alumina. The platinum provides the metal function, and brings about dehydrogenation and hydrogenation. The alumina provides the acidic function, being able to form carbocations from alkenes. The sequence of events in catalytic reforming shows up very clearly the complications that must be unravelled if a reaction as important as this is to be understood and improved. The first step is the attachment of the long-chain hydrocarbon by chemisorption to the platinum. In this process first one and then a second H atom is lost, and an alkene is formed. The alkene migrates to a Brønsted acid site, where it accepts a proton and attaches to the surface as a carbocation. This carbocation can undergo several different reactions. It can break into two, isomerize into a more highly branched form, or undergo varieties of ring-closure. Then the adsorbed molecule loses a proton, escapes from the surface, and migrates (possibly through the gas) as an alkene to a metal part of the catalyst where it is hydrogenated. We end up with a rich selection of smaller molecules which can be withdrawn, fractionated, and then used as raw materials for other products.
The concept of a solid surface has been extended with the availability of microporous materials, in which the surface effectively extends deep inside the solid. Zeolites are microporous aluminosilicates with the general formula {[Mn+]x/n·[H2O]m}{[AlO2]x[SiO2]y}x-, where Mn+ cations and H2O molecules bind inside the cavities, or pores, of the Al–O–Si framework (Fig. I22.1). Small neutral molecules, such as CO2, NH3, and hydrocarbons (including aromatic compounds), can also adsorb to the internal surfaces, accounting partially for the utility of zeolites as catalysts.
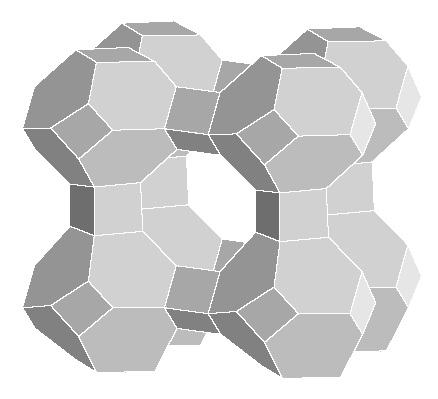
Figure I22.1 A framework representation of the general layout of the Si, Al, and O atoms in a zeolite material. Each vertex corresponds to a Si or Al atom and each edge corresponds to the approximate location of an O atom. Note the large central pore, which can hold cations, water molecules, or other small molecules.
Some zeolites for which M = H+ are very strong acids and catalyse a variety of reactions that are of particular importance to the petrochemical industry. Examples include the dehydration of methanol to form hydrocarbons such as gasoline and other fuels:
\(x\, \mathrm{CH_{3}OH \overset{zeolite}{\longrightarrow} (CH_{2})}_{x}+ x\, \mathrm{H_{2}O}\)
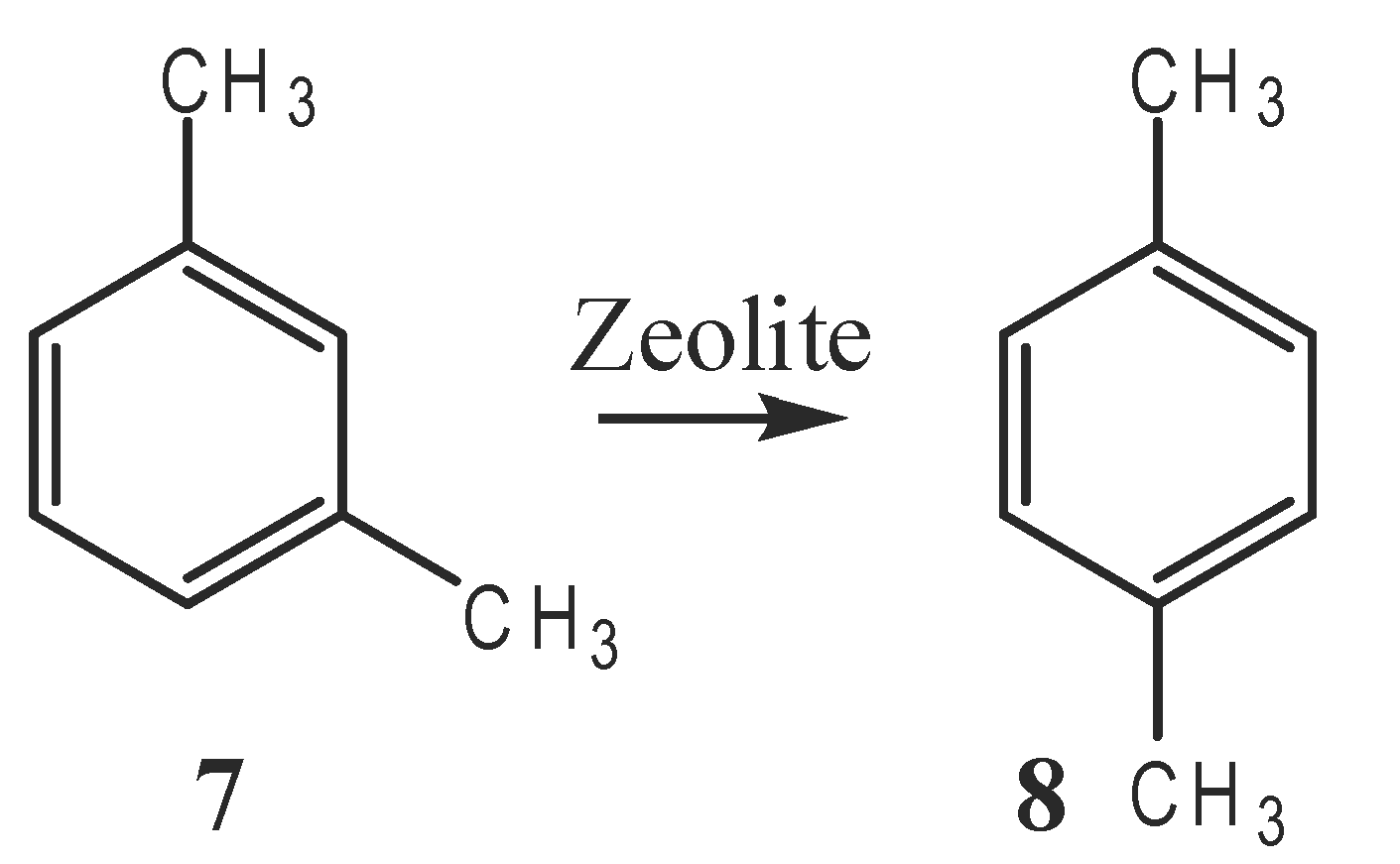
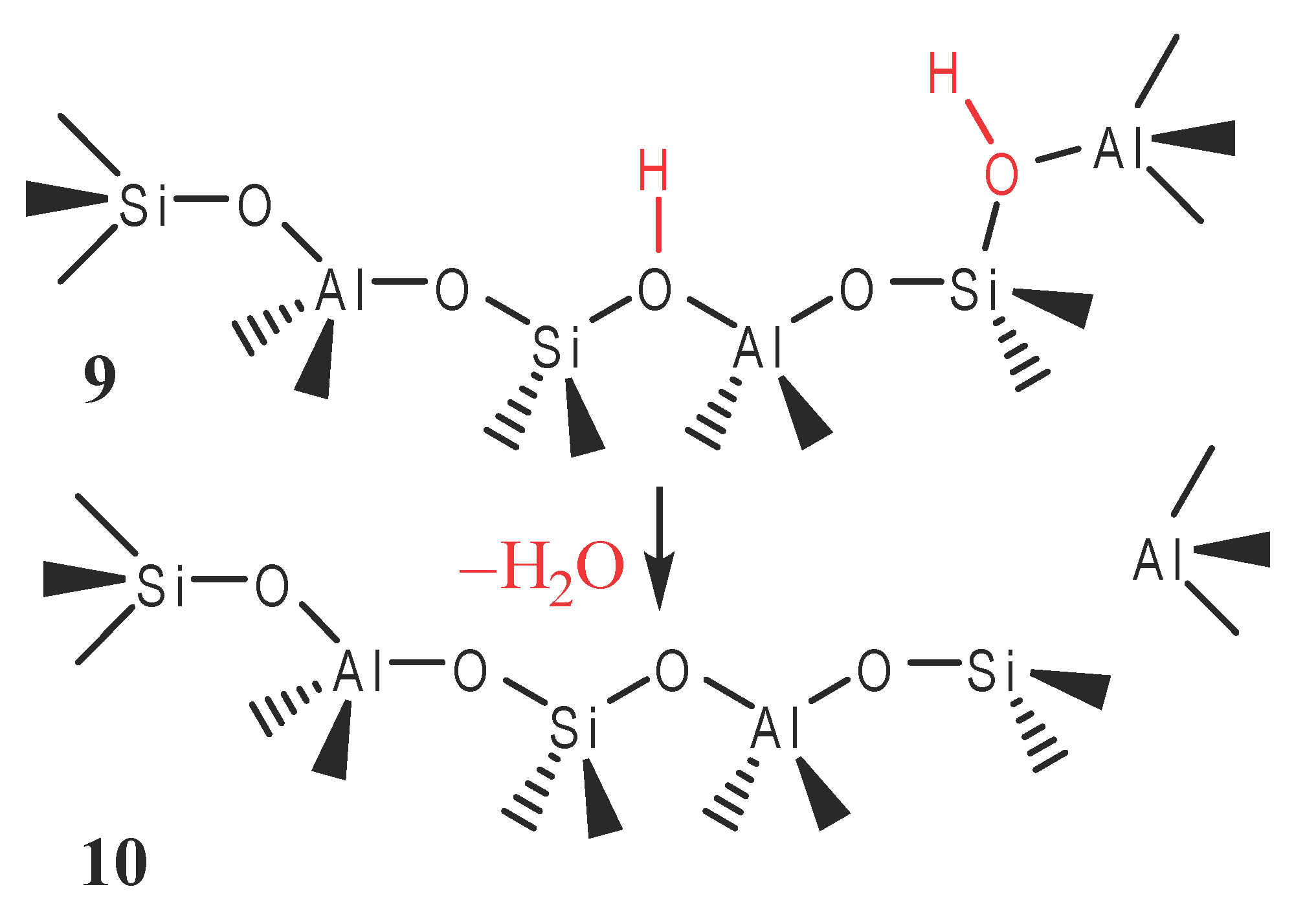
and the isomerization of m-xylene (7) to p-xylene (8). The catalytically important form of these acidic zeolites may be either a Brønsted acid (9) or a Lewis acid (10). Like enzymes, a zeolite catalyst with a specific composition and structure is very selective toward certain reactants and products because only molecules of certain sizes can enter and exit the pores in which catalysis occurs. It is also possible that zeolites derive their selectivity from the ability to bind and to stabilize only transition states that fit properly in the pores. The analysis of the mechanism of zeolite catalysis is greatly facilitated by computer simulation of microporous systems, which shows how molecules fit in the pores, migrate through the connecting tunnels, and react at the appropriate active sites.