Chapter 1. Impact 6.1
Impact…ON BIOCHEMISTRY: I6.1 Energy conversion in biological cells
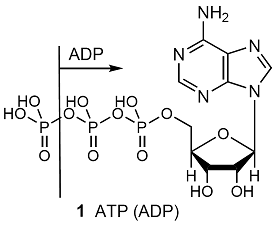
The whole of life’s activities depends on the coupling of exergonic and endergonic reactions, for the oxidation of food drives other reactions forward. In biological cells, the energy released by the oxidation of foods is stored in adenosine triphosphate (ATP, 1). The essence of the action of ATP is its ability to lose its terminal phosphate group by hydrolysis and to form adenosine diphosphate (ADP):
ATP(aq) + H2O(l) → ADP(aq) + Pi–(aq) + H3O+(aq)
Where Pi– denotes an inorganic phosphate group, such as H2PO4–. The biological standard values (Topic 5E) for ATP hydrolysis at 37 °C (310 K, blood temperature) are \(\Delta _{\mathtt{r}}G^{\oplus}\) = –31 kJ mol–1, \(\Delta_{\mathtt{r}}H^{\oplus}\) = –20 kJ mol–1, and \(\Delta_{\mathtt{r}}S^{\oplus}\) = +34 J K–1 mol–1. The hydrolysis is therefore exergonic (\(\Delta_{\mathtt{r}}G^{\oplus}\) < 0) under these conditions and 31 kJ mol–1 is available for driving other reactions. Moreover, because the reaction entropy is large, the reaction Gibbs energy is sensitive to temperature. In view of its exergonicity, the ADP–phosphate bond has been called a ‘high-energy phosphate bond’. The name is intended to signify a high tendency to undergo reaction, and should not be confused with ‘strong’ bond. In fact, even in the biological sense it is not of very ‘high energy’. The action of ATP depends on it being intermediate in activity. Thus ATP acts as a phosphate donor to a number of acceptors (for example, glucose), but is recharged by more powerful phosphate donors in a number of biochemical processes.
The oxidation of glucose to CO2 and H2O by O2 is an example of how the breakdown of foods is coupled to the formation of ATP in the cell. The process begins with glycolysis, a partial oxidation of glucose by nicotinamide adenine dinucleotide (NAD+, 2) to pyruvate ion, CH3COCO2–, continues with the citric acid cycle, which oxidizes pyruvate to CO2, and ends with oxidative phosphorylation, which reduces O2 to H2O. Glycolysis is the main source of energy during anaerobic metabolism, a form of metabolism in which inhaled O2 does not play a role. The citric acid cycle and oxidative phosphorylation are the main mechanisms for the extraction of energy from carbohydrates during aerobic metabolism, a form of metabolism in which inhaled O2 does play a role.
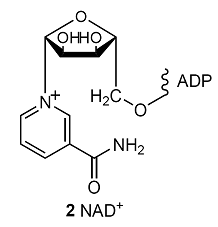
At blood temperature, \(\Delta_{\mathtt{r}}G^{\oplus}\) = –147 kJ mol–1 for the oxidation of glucose by NAD+ to pyruvate ions. The oxidation of one glucose molecule is coupled to the conversion of two ADP molecules to two ATP molecules, so the net reaction of glycolysis is
C6H12O6(aq) + 2 NAD+(aq) + 2 ADP(aq) + 2 Pi–(aq) + 2 H2O(l)
→ 2 CH3COCO2–(aq) + 2 NADH(aq) + 2 ATP(aq) + 2 H3O+(aq)
The standard reaction Gibbs energy is (–147) – 2(–31) kJ mol–1 = –85 kJ mol–1: the reaction is exergonic and can be used to drive other reactions.
The standard Gibbs energy of combustion of glucose is –2880 kJ mol–1, so terminating its oxidation at pyruvate is a poor use of resources. In the presence of O2, pyruvate is oxidized further during the citric acid cycle:
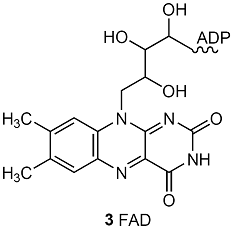
2 CH3COCO2–(aq) + 8 NAD+(aq) + 2 FAD(aq) + 2 ADP(aq) + 2 Pi–(aq) + 8 H2O(l)
→ 6 CO2(g) + 8 NADH(aq) + 4 H3O+(aq) + 2 FADH2(aq) + 2 ATP(aq)
where FAD is flavin adenine dinucleotide (3). The NADH and FADH2 go on to reduce O2 during oxidative phosphorylation, which also produces ATP. The citric acid cycle and oxidative phosphorylation generate as many as 38 ATP molecules for each glucose molecule consumed. Each mole of ATP molecules extracts 31 kJ from the 2880 kJ supplied by 1 mol C6H12O6 (180 g of glucose), so 1178 kJ is stored for later use. Therefore, aerobic oxidation of glucose is much more efficient than glycolysis.
In the cell, each ATP molecule can be used to drive an endergonic reaction for which \(\Delta_{\mathtt{r}}G^{\oplus}\) does not exceed +31 kJ mol–1. (In an actual cell, the composition may be far from standard and the ATP reaction much more potent.) For example, the biosynthesis of sucrose from glucose and fructose can be driven by plant enzymes because the reaction is endergonic to the extent \(\Delta_{\mathtt{r}}G^{\oplus}\) = +23 kJ mol–1. The biosynthesis of proteins is strongly endergonic, not only on account of the enthalpy change but also on account of the large decrease in entropy that occurs when many amino acids are assembled into a precisely determined sequence. For instance, the formation of a peptide link is endergonic, with \(\Delta_{\mathtt{r}}G^{\oplus}\) = +17 kJ mol–1, but the biosynthesis occurs indirectly and is equivalent to the consumption of three ATP molecules for each link. In a moderately small protein like myoglobin, with about 150 peptide links, the construction alone requires 450 ATP molecules, and therefore about 12 mol of glucose molecules for 1 mol of protein molecules.