All fats have two distinct components: they have a “head” region and two or three long “tails” (FIGURE 2-29). The head region is a small molecule called glycerol. It is linked to “tail” molecules known as fatty acids. A fatty acid is simply a long hydrocarbon—
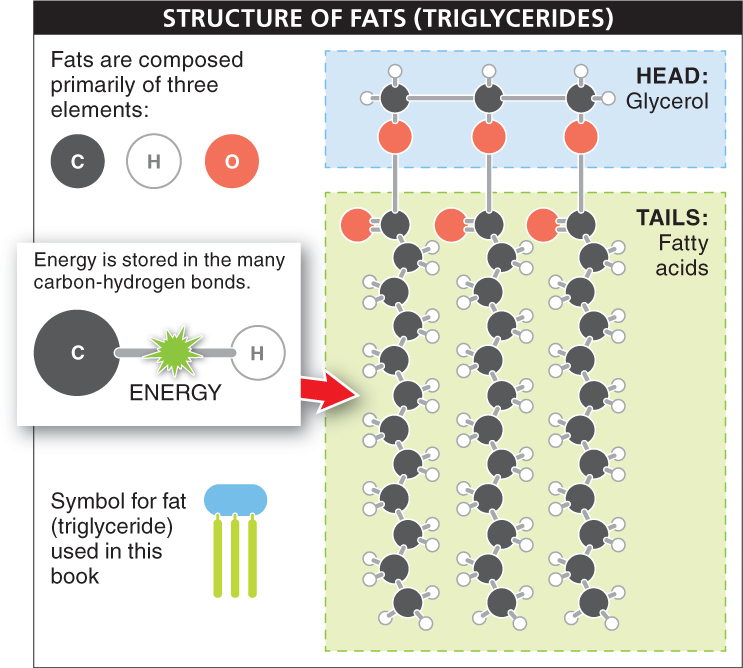
The fats in most foods we eat are triglycerides, which are fats having three fatty acids linked to the glycerol molecule. For this reason, the terms “fats” and “triglycerides” are often used interchangeably. Triglycerides that are solid at room temperature are generally called “fats,” while those that are liquid at room temperature are called “oils.”
Fat molecules contain much more stored energy than do carbohydrate molecules. That is, the chemical breakdown of fat molecules releases significantly more energy. A single gram of carbohydrate stores about 4 calories of energy, while the same amount of fat stores about 9 calories—

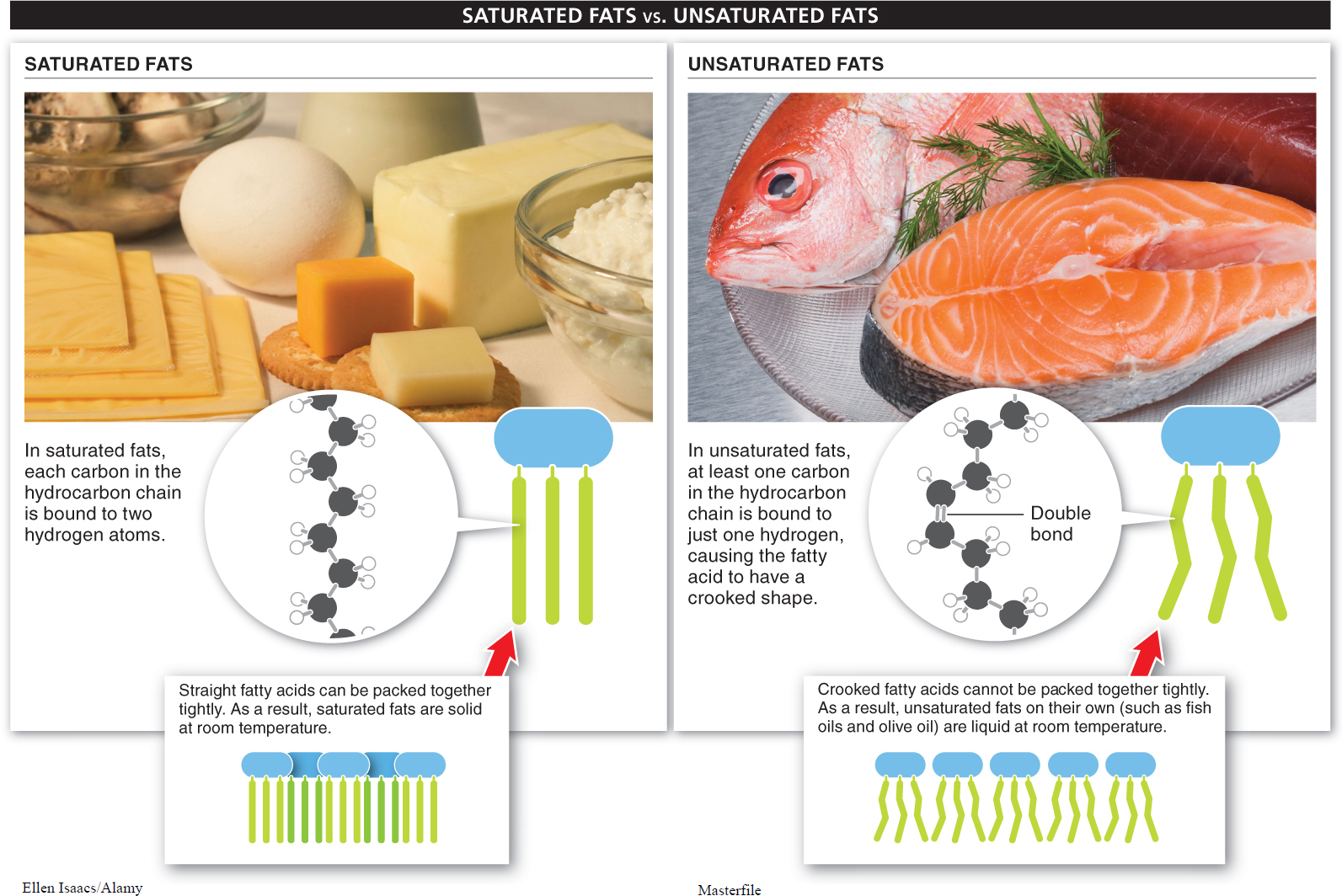
An important distinction is made between “saturated” and “unsaturated” fats (FIGURE 2-31). These terms refer to the hydrocarbon chain in the fatty acids. If each carbon atom in the hydrocarbon chain of a fatty acid is bonded to two hydrogen atoms, the fat molecule carries the maximum number of hydrogen atoms and is said to be a saturated fat. Most animal fats, including those found in meat and eggs, are saturated. They are not essential to your health and, because they accumulate in your bloodstream and can narrow the vessel walls, they can contribute to heart disease and strokes.
63
64
An unsaturated fat is one in which some of the carbon atoms are bound to only a single hydrogen (and are connected to each other by a double bond). Most plant fats are unsaturated. Unsaturated fats may be mono-
How will the “chewy-
The shapes of unsaturated fat molecules and saturated fat molecules are different. When saturated, the hydrocarbon tails of the fatty acids all line up very straight and the fat molecules can be packed together tightly. The tight packing causes the fats, such as butter, to be solid at room temperature. When unsaturated, the fatty acids have kinks in the hydrocarbon tails and the fat molecules cannot be packed together as tightly (see Figure 2-
The ingredient list for many snack foods includes “partially hydrogenated” vegetable oils. The hydrogenation of an oil means that hydrogen atoms have been added to a liquid, unsaturated fat so that it becomes more saturated. Adding hydrogen atoms can create a food with a more desirable texture, since increasing a fat’s degree of saturation changes its consistency and makes it more solid at room temperatures. By attaining just the right degree of saturation, it is possible to create foods, such as chocolate, that are near the border of solid and liquid and “melt in your mouth” (FIGURE 2-32). Unfortunately, hydrogenation also makes the food less healthful because saturated fats increase the risk of heart disease. They are less reactive—

Hydrogenation of unsaturated fats is doubly problematic from a health perspective because it also creates trans fats, the “trans” referring to the unusual orientation of some or all of the double bonds that remain following the addition of hydrogen atoms. This orientation differs from that in other unsaturated dietary fats—
65
Olestra is a recently developed “fake fat” chemical that gives foods the taste of fat, without adding the calories of fats. What chemical structure might make this possible?
Because of the well-
TAKE-HOME MESSAGE 2.13
Fats, including the triglycerides common in the food we eat, are one type of lipid. Characterized by long hydrocarbon tails, fats effectively store energy in the many carbonhydrogen and carbon-
What two types of molecules are combined to make a fat or oil?
A triglyceride consists of a single glycerol molecule covalently linked to three fatty acids. Triglycerides that are solid at room temperature are called fats, and triglycerides that are liquid at room temperature are called oils.