A little bit of chemistry goes a long way in the study of biology and in understanding a great deal about your everyday life. Will eating those beans in your soup keep you up all night in gastrointestinal distress? Just knowing whether the beans are lentils or lima beans—
The chemistry that is most important in biology revolves around a few important elements, which are introduced at the end of this section. An element is a substance that cannot be broken down chemically into any other substances. Gold, carbon, and copper are elements you might be familiar with. Whatever the element, if you keep cutting it into ever smaller pieces, each of the pieces behaves exactly the same as any other piece. The smallest piece of pure gold will still have the softness, reflectivity, and malleability characteristic of that element (FIGURE 2-1).

If you could continue cutting, you would eventually separate the gold into tiny pieces that could no longer be divided without losing their gold-
41
Everything around us, living or not, can be reduced to atoms. All atoms—
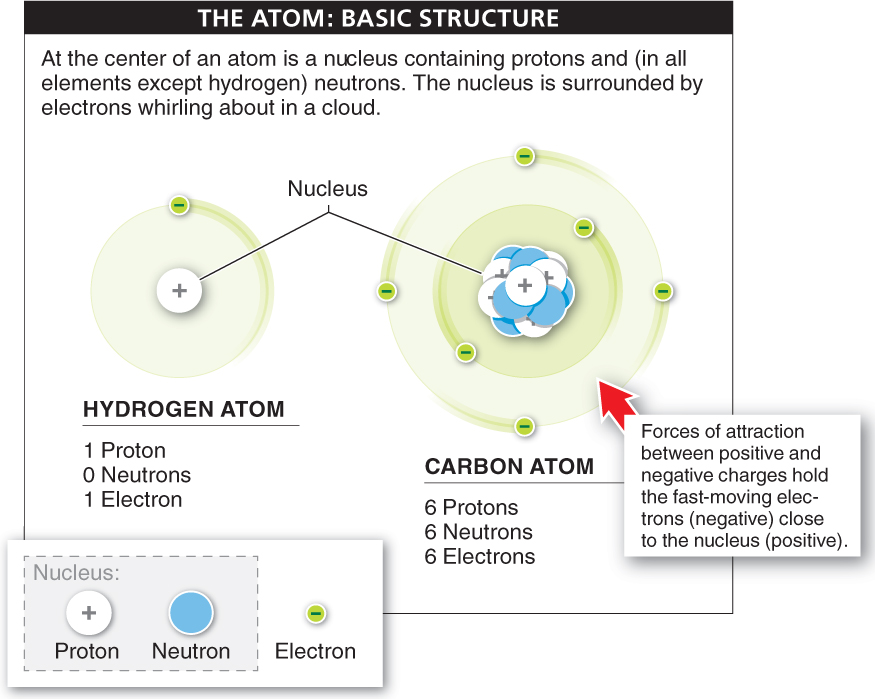
Whirling in a cloud around the nucleus of every atom are negatively charged particles called electrons. An electron weighs almost nothing—
Particles that have the same charge repel each other; those with opposite charges are attracted to each other. Because all electrons have the same charge, the electrons in an atom repel each other. But because they are negatively charged, they are attracted to the positively charged protons in the nucleus. This attraction holds electrons close enough to the nucleus to keep them from flying away, while the energy of their fast movement keeps them from collapsing into the nucleus. When the number of protons and electrons is equal, the charges in the atom are balanced.
Atoms are tiny. Enlarge an atom by a billion times and it would only be the size of a grapefruit. Paradoxically, most of the space taken up by an atom is empty. That is, because the nucleus is very small and compact, the electrons zip about relatively far from the nucleus. If the nucleus were the size of a golf ball, the electrons would be anywhere from half a mile to six miles away.
Elements differ in their number of protons What distinguishes one element, such as chlorine, from another, such as neon or oxygen? The number of protons in an atom’s nucleus determines what element it is. As a rule, atoms of different elements have a different number of protons in the nucleus. A chlorine atom has 17 protons, a neon atom has 10 protons, and an oxygen atom has 8 protons. Each element is given a name (and an abbreviation, such as O for oxygen and C for carbon) and an atomic number that corresponds to how many protons it has (FIGURE 2-3).

The mass of an atom is often about double the element’s atomic number. This is the case when the number of neutrons in the nucleus is equal to the number of protons, because protons and neutrons have approximately the same mass. The element oxygen, for example, has the atomic number 8 reflecting that it has 8 protons, and because it has 8 neutrons it has an atomic mass of 16, simply the mass of the 8 protons and the mass of the 8 neutrons added together. (As noted above, we can ignore the mass of the electrons.)
42
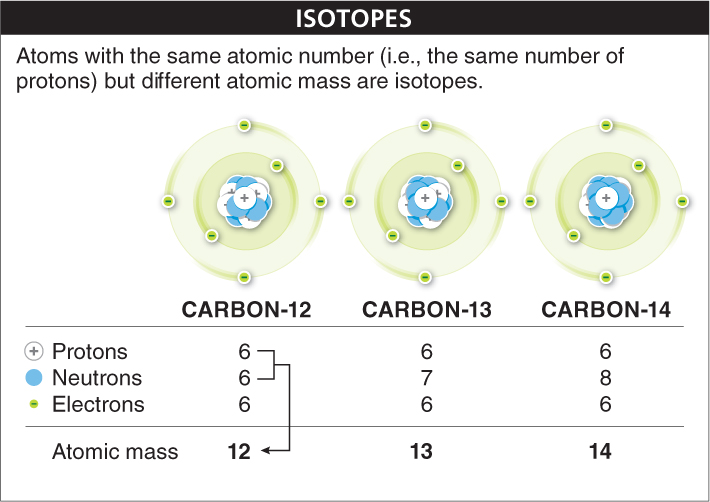
Atoms of the same element don’t always have the exact same number of neutrons in their nucleus and electrons circling around it. They sometimes acquire or lose components. For example, an atom may have extra neutrons or fewer neutrons than the number of protons. Atoms with the same number of protons but different numbers of neutrons are called isotopes. An atom’s charge doesn’t change in an isotope, because neutrons have no electrical charge, but the atom’s mass changes with the loss or addition of another particle in the nucleus (FIGURE 2-4). Carbon, for example, has six protons and so usually has a mass of 12. Occasionally, though, a rare carbon atom has an extra neutron or two and an atomic mass of 13 or 14. These isotopes are called carbon-
Most elements and their isotopes have perfectly stable nuclei that remain unchanged virtually forever, never losing or gaining neutrons, protons, or electrons. A few atomic nuclei are not so stable, however, and break down spontaneously sometime after they are created. These atoms are radioactive, and in the process of decomposition they release, at a constant rate, a tiny, high-
All the known elements can be arranged in a scheme, in the order of their atomic number, called the periodic table (see Figure 2-
43
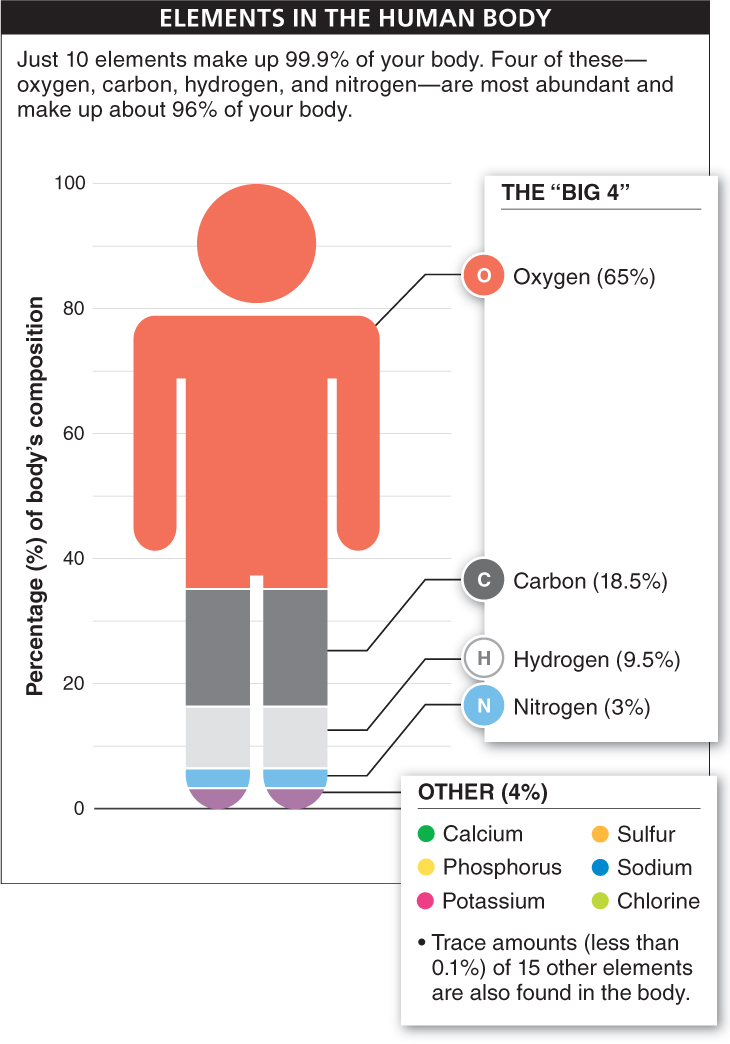
Of all the elements found on earth, only 25 are found in your body. The “Top 10” most common of these make up 99.9% of your body mass, but they are not present in equal measures (FIGURE 2-5). The “Big 4” elements—
TAKE-HOME MESSAGE 2.1
Everything around us, living or not, is made up of atoms, the smallest unit into which material can be divided without losing its essential properties. All atoms have the same general structure. They are made up of protons and neutrons in the nucleus, and electrons, which circle far and fast around the nucleus.
How does one determine the atomic number and the atomic mass of an element?
The atomic number is the number of protons in an atom’s nucleus. The atomic mass is the combined mass of an atom’s protons and neutrons.