Protein shape is particularly critical in enzymes, molecules that help initiate and accelerate the chemical reactions in our bodies. Enzymes emerge unchanged—
Think of an enzyme as a big piece of popcorn. Its tertiary or quaternary structure gives it a complex shape with lots of nooks and crannies. Within one of those nooks is a small area called the active site (FIGURE 2-42). Based on the chemical properties of the atoms lining this pocket, the active site provides a place for the participants in a chemical reaction, the reactants or substrate molecules, to nestle briefly.
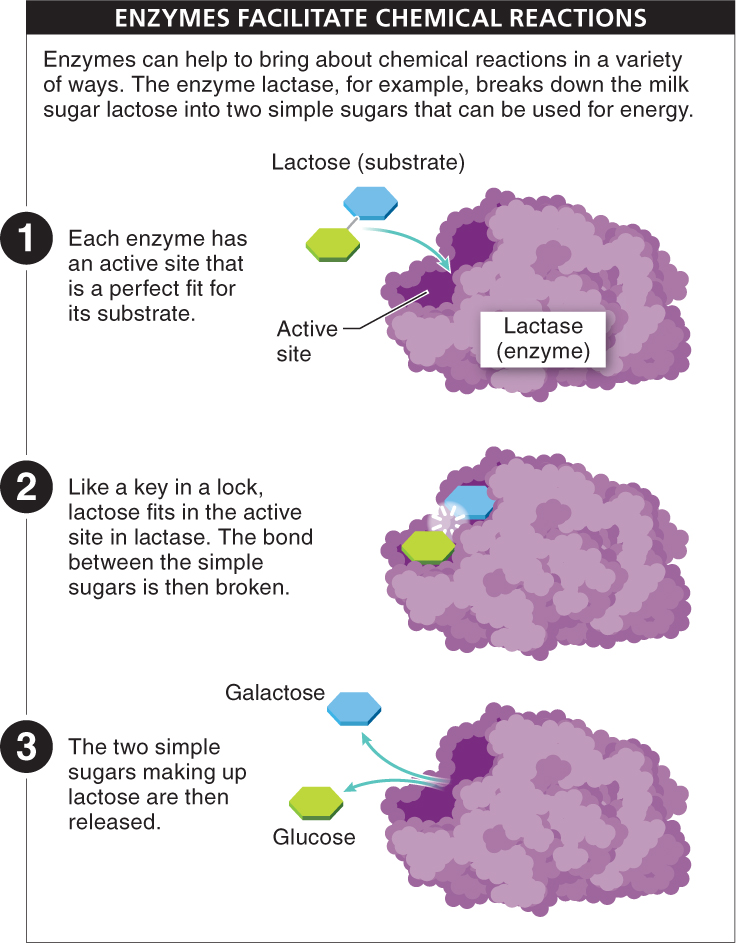
Enzymes are very choosy: they bind only with their appropriate substrate molecules, much like a lock that can be opened with only one key (see Figure 2-
The chemical reactions that occur in organisms can either release energy or consume energy. But in either case, a certain minimum energy—
By virtue of their catalytic capacities, enzymes are at the heart of the chemistry of living organisms. Taken together, all of the chemical reactions in a living organism are its metabolism.
Increasingly complex molecules are synthesized or degraded in a series of sequential reactions called a “metabolic pathway.” Each step of these metabolic pathways is catalyzed by an enzyme produced in the body. Proteins are the building blocks with which living organisms are built, but since nearly all enzymes are proteins, proteins can also be thought of as the builders of bodies, too.
TAKE-HOME MESSAGE 2.18
Enzymes are proteins that help initiate and speed up chemical reactions. They aren’t permanently altered in the process, but rather can be used again and again.
How would you define an enzyme? Discuss the steps by which enzymes facilitate chemical reactions.
Enzymes are proteins that help initiate and speed up chemical reactions. Enzymes are not used up in the reaction, nor are they changed. Thus, they can be used over and over again. Enzymes have an active site that binds to a specific substrate, much like one key fits a specific lock. In a metabolic pathway, enzymes bind to the substrate at the active site, positioning the atoms in the active site in a manner that weakens the bonds in the substrate, lowering the activation energy necessary for the chemical reaction to occur. Depending upon the reaction, bonds are either broken or formed, and products are released from the active site of the enzyme. The enzyme is unchanged, available to catalyze more reactions.
73