If not for enzymes, it is possible that nothing would ever get done. At least not in living organisms. Enzymes don’t alter the outcome of reactions, but without the chemical “nudge” they supply—
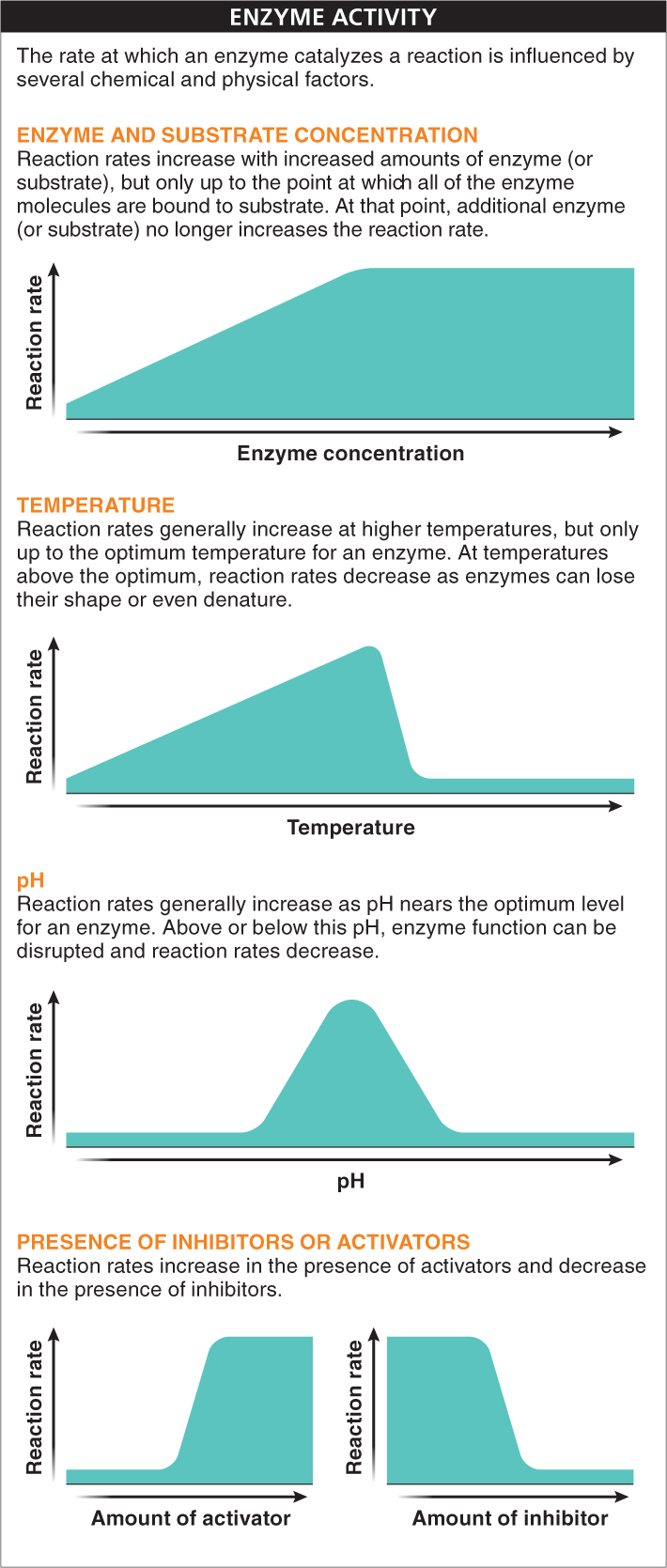
The rate at which an enzyme catalyzes a reaction is influenced by several chemical and physical factors (FIGURE 2-43). These include:
- 1. Enzyme and substrate concentration. For a given amount of substrate, an increase in the amount of enzyme increases the rate at which the reaction occurs. Similarly, for a given amount of enzyme, an increase in the substrate concentration increases the reaction rate. In both cases, once all of the enzyme molecules are bound to substrate, or vice versa, additional enzyme or substrate no longer increases the reaction rate.
- 2. Temperature. Because increasing the temperature increases the speed of movement of molecules, reaction rates generally increase at higher temperatures. Reaction rates continue to increase only up to the optimum temperature for an enzyme. At temperatures above the optimum, reaction rates decrease as enzymes lose their shape or even denature. Enzymes from different species can have widely differing optimum temperatures.
- 3. pH. As with temperature, enzymes have an optimum pH. Above or below this pH, excess hydrogen or hydroxide ions interact with amino acid side chains in the active site. These interactions disrupt enzyme function (and sometimes structure) and decrease reaction rates.
- 4. Presence of inhibitors or activators. One of the most common ways that cells can speed up or slow down their metabolic pathways is through the binding of other chemicals to enzymes. This binding can alter enzyme shape in a way that increases or decreases the enzyme’s activity. Inhibitors reduce enzyme activity and come in two types. Competitive inhibitors bind to the active site, blocking substrate molecules from the site and thus from taking part in the reaction. Noncompetitive inhibitors do not compete for the active site but, rather, bind to another part of the enzyme, altering its shape in a way that changes the structure of the active site, thus reducing or blocking its ability to bind with substrate. Often, it is the very product of a metabolic pathway that acts as an inhibitor of enzymes early in the pathway, effectively shutting off the pathway when enough of its end product has been produced.
74
Just as a molecule can bind to an enzyme and inhibit the enzyme’s activity, so can some cellular chemicals act as activators. Instead of their binding to the enzyme “turning it off,” their binding to the enzyme “turns it on,” altering the enzyme’s shape or structure so that it can now catalyze a reaction.
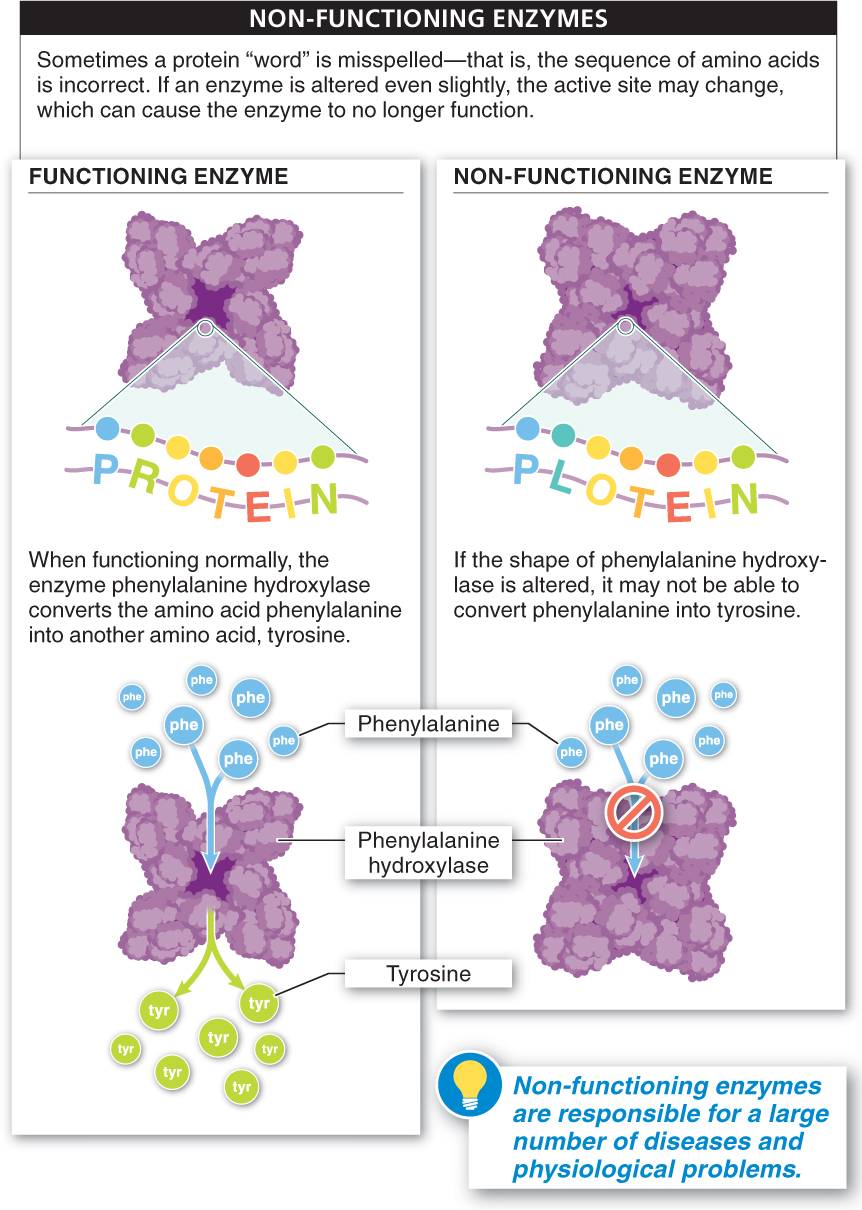
Sometimes a cell produces a protein “word” that is misspelled—
Why do some adults get sick when they drink milk?
One health issue influenced by enzyme function is the condition called lactose intolerance. Normally, during digestion, the lactose in milk is broken down into its component parts, glucose and galactose (see Figure 2-
The unpleasant symptoms of lactose intolerance can be avoided by not consuming milk, cheese, yogurt, ice cream, or any other dairy products, but they can also be avoided by taking a pill containing the enzyme lactase. It doesn’t matter how the enzyme gets into your digestive system; as long as it’s there, the lactose in the milk can be broken down.
TAKE-HOME MESSAGE 2.19
Enzyme activity is influenced by physical factors such as temperature and pH, as well as by chemical factors, including enzyme and substrate concentrations. Inhibitors and activators are chemicals that bind to enzymes and, by blocking the active site or altering the shape or structure of the enzyme, can change the rate at which the enzyme catalyzes reactions.
Discuss the various factors that influence the rate of enzymatic reactions. Explain what can happen if an enzyme is altered even slightly.
The main physical and chemical factors that influence the rate at which an enzyme catalyzes a reaction include: enzyme and substrate concentration, temperature, pH, salinity, and the presence or absence of inhibitors or activators. Higher concentrations of enzyme and/or substrate increase the reaction rate (up to a point). Inhibitor concentrations lower the reaction rate, whereas activator concentrations speed up the reaction rate. Alterations in temperature, pH, and salinity also can affect the reaction rate by speeding it up, slowing it down, or stopping it altogether. If an enzyme is altered even slightly, the active site may change, and the enzyme may cease to function.
75