
Every so often, a tadpole or small fish gets an unexpected—
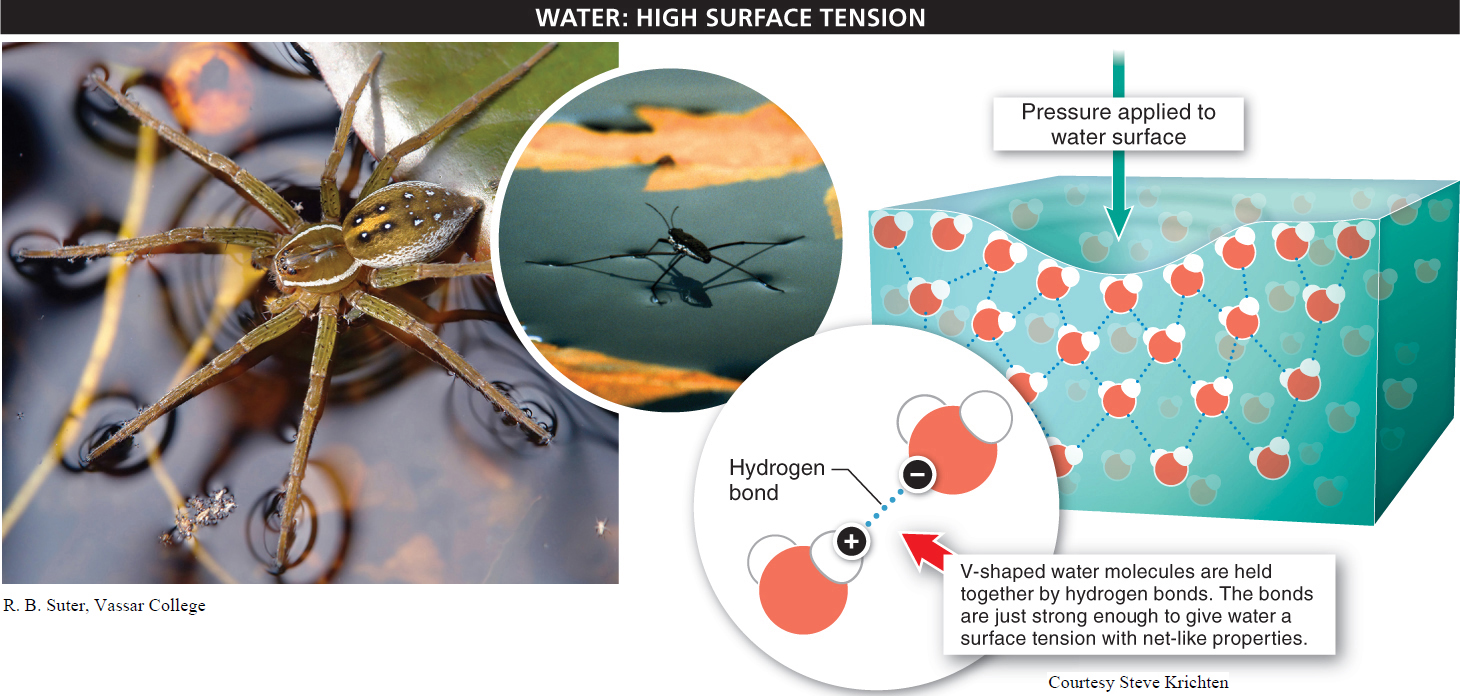
The fishing spider, like numerous other insects such as the water strider, makes use of the fact that water molecules have tremendous cohesion. That is, they stick together with unusual strength. This molecular cohesiveness is due to hydrogen bonds between the water molecules.
Each water molecule is V-
49
Because of their unequally shared electrons, water molecules are polar (see Figure 2-
Hydrogen bonds, as we’ve noted, are much weaker than covalent and ionic bonds, and they don’t last very long. Nonetheless, to the fishing spider, the cumulative effect of all the hydrogen bonds in water is to link together all the water molecules in the stream just enough to give the water a surface tension with some net-
TAKE-HOME MESSAGE 2.4
Water molecules easily form hydrogen bonds, giving water great cohesiveness.
Describe how the polar covalent bonds within water molecules lead to the formation of hydrogen bonds between water molecules.
Water is held together by polar covalent bonds, with an oxygen atom having a partial negative charge and hydrogen atoms having partial positive charges. A hydrogen bond is an attractive force between the oxygen atom of one water molecule and a hydrogen atom of another water molecule. Because each water molecule can participate in multiple hydrogen bonds, this makes water molecules stick together and resist being separated.