All life on earth depends on water; organisms are made up mostly of water and require it more than any other molecule. Hydrogen bonding among water molecules gives water several important properties that contribute to its crucial role in the biology of all organisms.
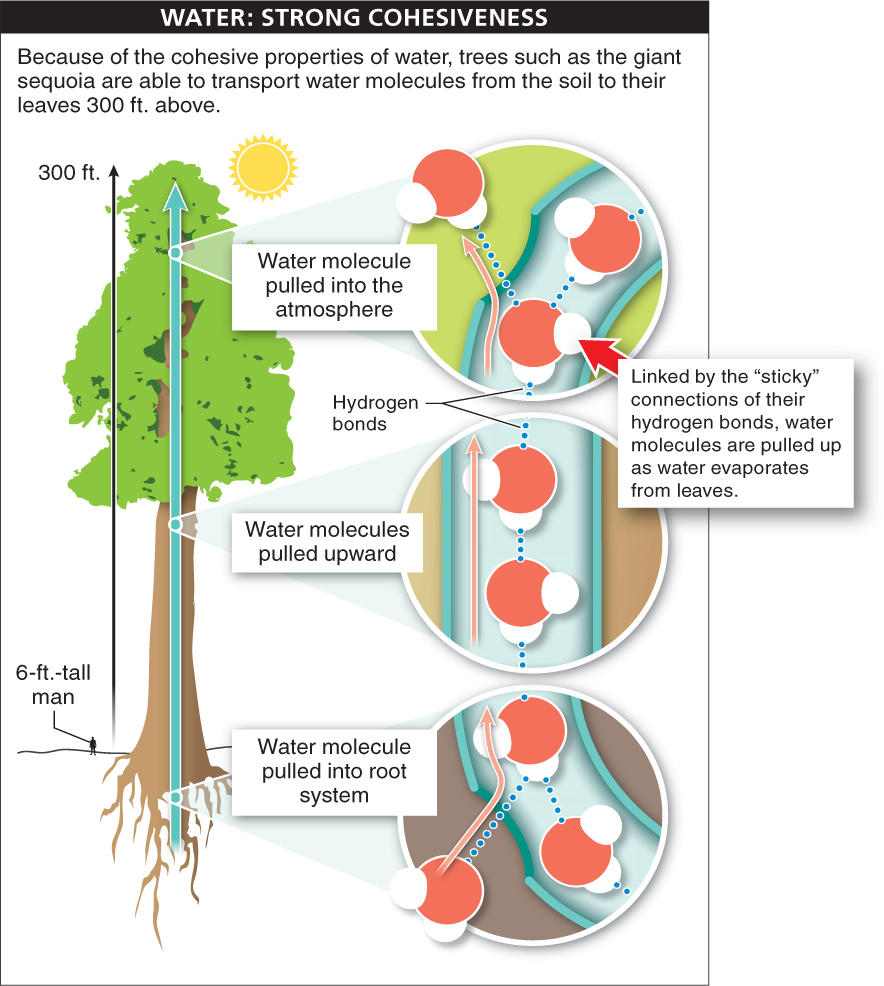
1. Cohesion. We saw in the previous section how the connection of water molecules through hydrogen bonds makes water cohesive, resulting in, for example, high surface tension. The cohesiveness of water molecules also makes it possible for tall trees to exist (FIGURE 2-14). Leaves need water. Molecules of water in the leaves are continually lost to the atmosphere through evaporation or are used up in the process of photosynthesis. To get more water, plants must pull it up from the soil. The problem for many plants, such as the giant sequoia trees, is that the soil may be 300 feet below the leaf. However, water molecules can pull up adjacent water molecules to which they have hydrogen-
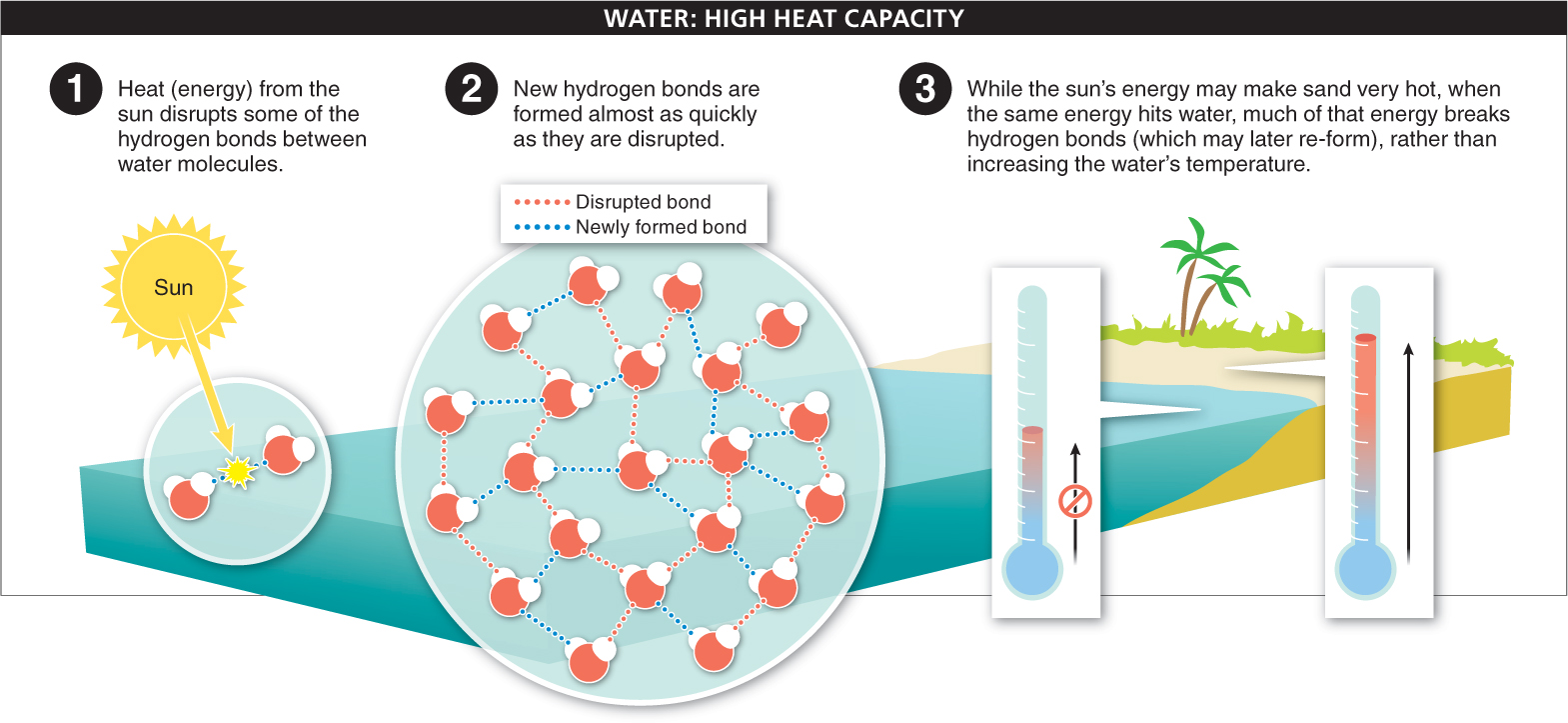
2. Large heat capacity. Walking across a sandy beach on a hot day, you can feel how easily sand heats up. By comparison, the water is cool as you step from the beach into the ocean, because water resists warming. It takes a lot of energy to change the temperature of water even a small amount. Why? Again, we must look to hydrogen bonding for our answer.
The temperature of a substance is a measure of how quickly all of the molecules are moving. The molecules move more quickly when energy is added in the form of heat. When we heat water, the added energy doesn’t immediately increase the movement of the individual water molecules. Rather, it disrupts some of the hydrogen bonds between the molecules (FIGURE 2-15). As quickly as they can be disrupted, though, hydrogen bonds form again somewhere else. And since the water molecules themselves don’t increase their movement, the temperature doesn’t increase. The net effect is that even if you release a lot of energy into water, the temperature doesn’t change much. For this reason, because so much of your body is water, you are able to maintain a relatively constant body temperature.
50
Why do coastal areas have milder, less variable climates than inland areas?
Large bodies of water, especially oceans, can absorb huge amounts of heat from the sun during warm times of the year, reducing temperature increases on the coastland. During cold times of the year, the ocean cools slowly, giving off heat that reduces the temperature drop on shore.
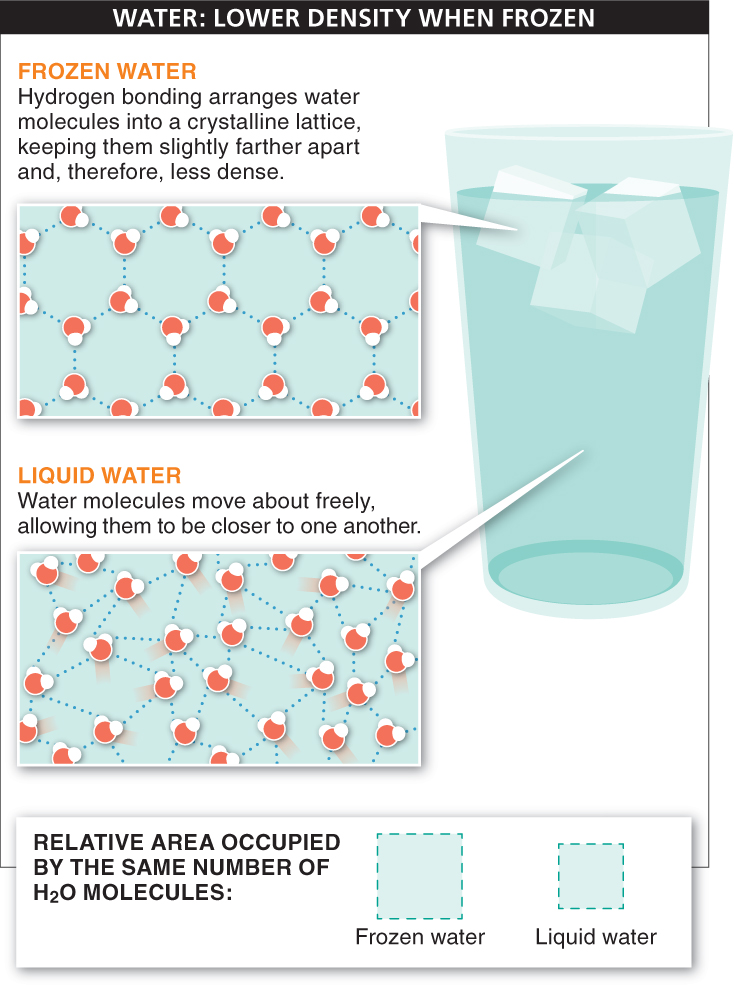
3. Low density as a solid. Ice floats. This is unusual because most substances increase in density when frozen. As the cooling molecules slow down, they pack together more and more efficiently—
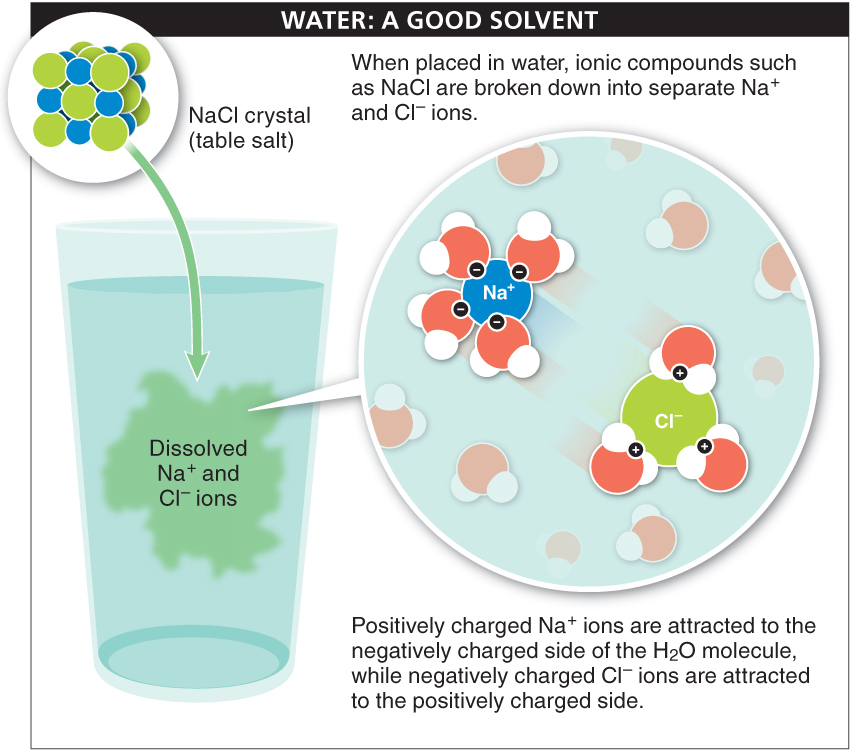
4. Good solvent. If you put a pinch of table salt into a glass of water, it will quickly dissolve. This means that all the charged sodium (Na+) and chloride (Cl−) ions that were ionically bonded together become separated from one another. The sodium and chloride ions were initially attracted to each other because they carry a slight opposing charge. Water is able to pry them apart because, as a polar molecule, it, too, carries charges. The positively charged sodium ions are attracted to the negatively charged side of the water molecule, and the negatively charged chloride ions are attracted to the positively charged side (FIGURE 2-17). The ionic bonds holding together the ions are broken, and each ion becomes surrounded by water moleculses. Many substances are, like water, polar and consequently dissolve easily in water.
51
Nonpolar molecules (such as oils) have neither positively charged regions nor negatively charged regions. Consequently, the polar water molecules are not attracted to them. Instead, when an oil is poured into a container of water, the oil molecules remain in clumps that never dissolve.
Why don’t oceans freeze as easily as freshwater lakes?
Because so much salt is dissolved in the oceans, many of the water molecules have their positively charged sides facing Cl− ions and, simultaneously, many molecules of water have their negatively charged sides facing Na+ ions. Consequently, the orderly lattices of hydrogen bonds found in ice cannot form in salt water, and it does not freeze well.
TAKE-HOME MESSAGE 2.5
The hydrogen bonds between water molecules give water several of its most important characteristics, including cohesiveness, reduced density as a solid, the ability to resist temperature changes, and broad effectiveness as a solvent for ionic and polar substances.
List the four key properties of water conferred by its ability to form hydrogen bonds.
Water is cohesive, has a large heat capacity, has a lower density as a solid than as a liquid, and is a powerful solvent.