There’s a lot more going on in water than meets the eye. Most of the molecules are present as H2O, but at any instant some of them break into two parts: H+ and OH−. In pure water, the amount of H+ and OH− must be exactly the same, since every time a molecule splits, one of each type of ion is produced. But in some fluids containing other dissolved materials, this balance is lost: the fluid can have more H+ or more OH−.
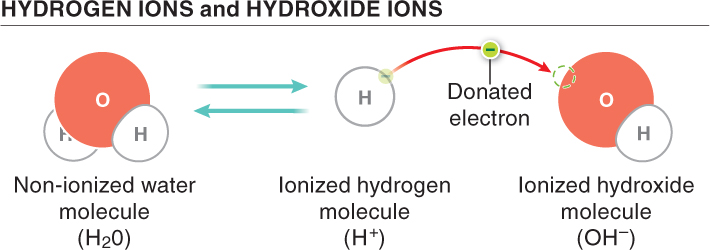
The amount of H+ or OH− in a fluid gives it some important properties. In particular, the amount of H+ in a solution is a measure of its acidity and is called pH. The greater the number of free hydrogen ions floating around, the more acidic the solution is.
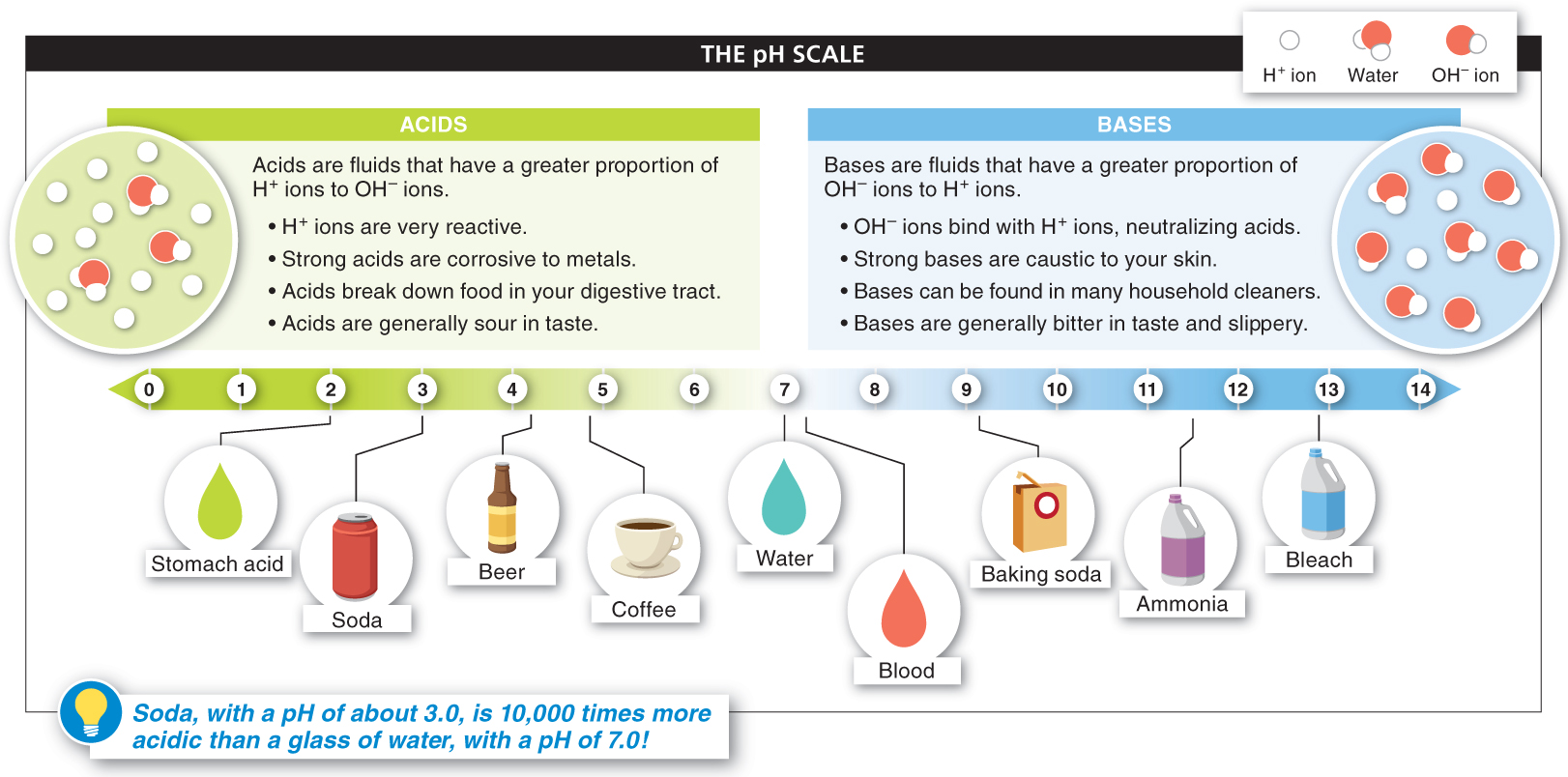
Pure water is in the middle of the pH scale, with a pH of 7.0. Any fluid with a pH below 7.0 has more H+ ions (and fewer OH− ions) and is considered an acid. Any fluid with a pH above 7.0 has fewer H+ ions (and more OH− ions) and is considered a base. The pH scale, like the Richter scale for earthquakes, is logarithmic, although in the case of pH, the lower the number the greater the acidity: a decrease of 1 on the pH scale represents a 10-
52
H+ ions are essentially free-
Is acid rain likely to have any impact on populations of microbes living in lakes, streams, and soil? Why?
Your stomach produces large amounts of hydrochloric acid (HCl dissolved in water) and has a pH between 1 and 3. (HCl dissolved in water is acidic because most of the hydrogen ions split off from the chlorine, raising the H+ concentration of the fluid.) The acid in your stomach helps to kill most bacteria that you ingest. It also greatly enhances the breakdown of the chemicals in the food you eat and the efficiency of digestion and absorption. You may have learned firsthand of the high acidity of your stomach fluids if you have experienced heartburn or the sour taste of vomit.
Acids have a higher concentration of H+ than of OH−. Conversely, bases have a higher concentration of OH− than of H+. Baking soda is a common basic substance. Some bases are called “antacids” because the OH− ions in bases can bind with excess H+ ions in acidic solutions, neutralizing the acid. Base-
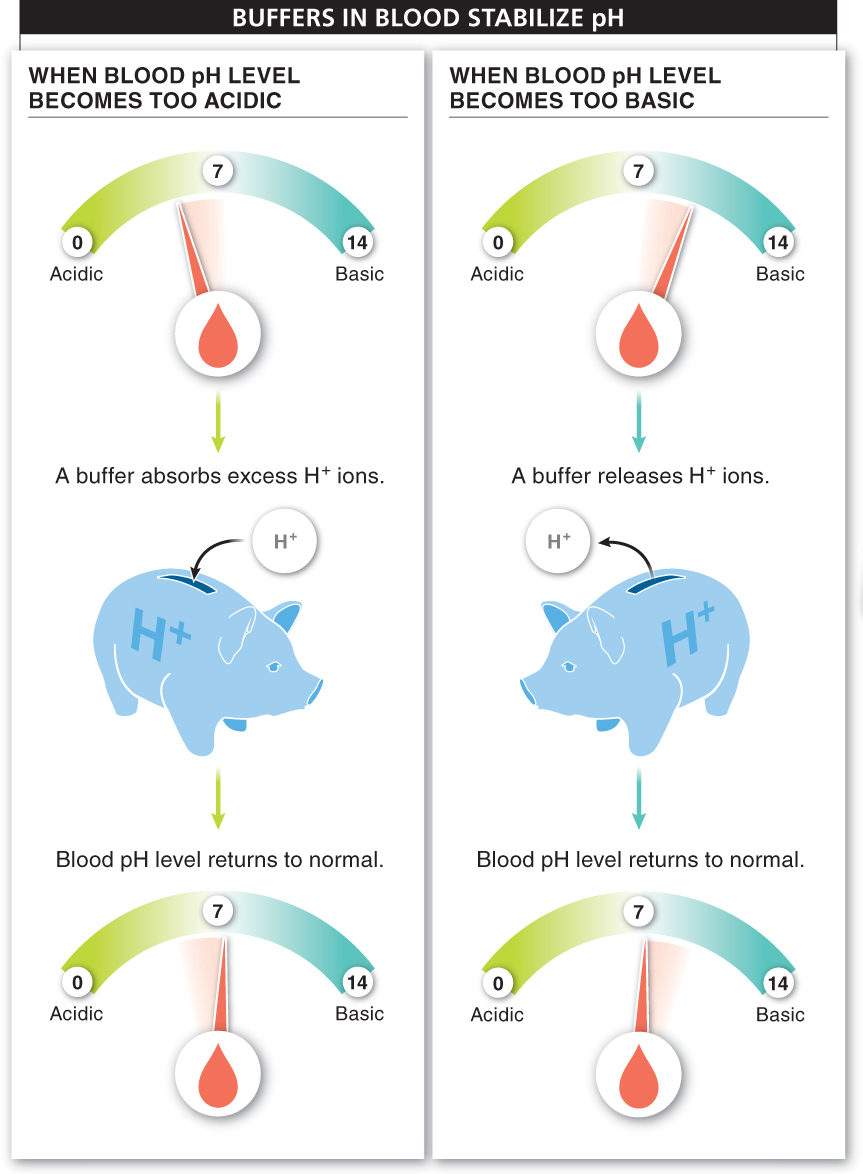
The pH of blood is usually 7.4. Given that most cellular reactions produce or consume H+ ions, you might expect there to be great swings in the pH of our blood. Unfortunately, our bodies can’t tolerate such swings. Most of the chemical reactions in our blood or cells stop proceeding properly if the pH swings up or down, even by less than half a point. Fortunately, our bodies contain some chemicals that act like bank accounts for H+ ions (FIGURE 2-19). Called buffers, these chemicals can quickly absorb excess H+ ions to keep a solution from becoming too acidic, and they can quickly release H+ ions to counteract any increases in OH− concentration. Buffers are chemicals that act to resist changes in pH.
53
TAKE-HOME MESSAGE 2.6
The pH of a fluid is a measure of how acidic or basic the solution is and depends on the concentration of dissolved H+ ions present; the lower the pH, the more acidic the solution. Acids, such as vinegar, can donate protons to other chemicals; bases, including baking soda, bind with free protons.
Why wouldn’t your blood and other fluids become significantly acidic after you drink a half gallon of orange juice?
Although the orange juice is acidic, your body contains buffers that absorb these excess hydrogen ions and preserve the neutral pH of your blood.